Taxol-based small-molecule hydrogel and preparation method thereof
A technology of small molecule water and paclitaxel, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas. Improvement effect, the effect that the composition method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Synthesis of Taxol-SA (referring to literature J.Med.Chem., 1989, 32 (4), pp 788-792), the structural formula of Taxol-SA is as follows:
[0020]
[0021] (2) Synthesis of Taxol-SA-GSSG (compound 1), the steps are as follows:
[0022]
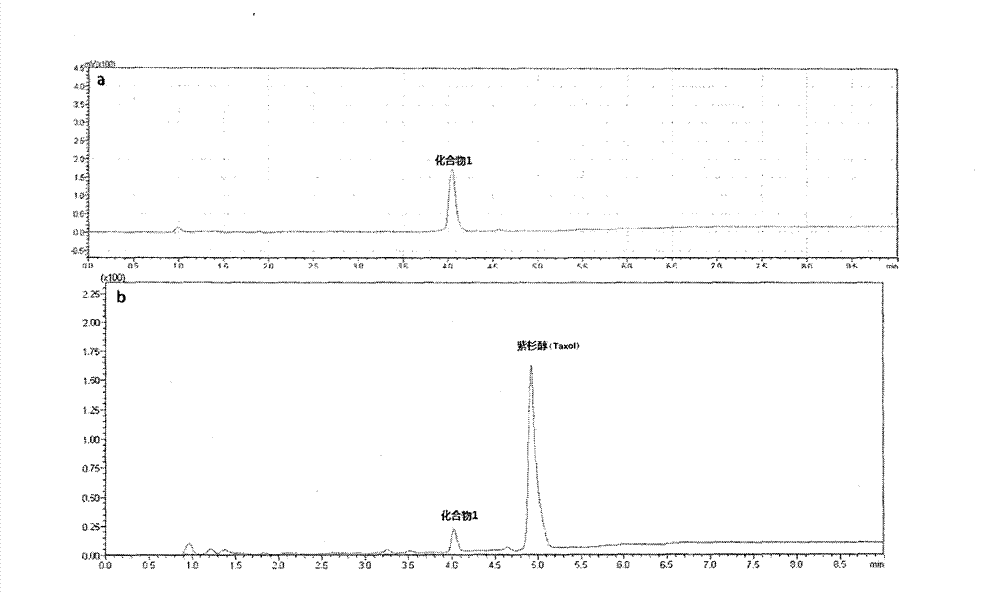
[0023] Weigh 100 mg (104.8 μmol) Taxol-SA, dissolve it in 20 mL of anhydrous dichloromethane (DCM), and add the following substances in sequence: 13.3 mg (115.3 μmol) N-hydroxysuccinimide (NHS) and 25.7 mg (125.4 μmol) N, N'-dicyclohexylcarbodiimide (DCC), stirred at room temperature in the dark for three hours, filtered, and the filtrate was collected and spin-dried. Add 10mL of acetone (Acetone), and at the same time add 6mL of an aqueous solution of oxidized glutathione (GSSG) (the content of oxidized glutathione is 96.2mg, add NaHCO 3 About 40 mg was adjusted to pH=7.4), reacted in the dark for 12 hours, spin-dried in acetone, then freeze-dried, separated by high-performance liquid chromatography, and the yield was 83.2%. ...
Embodiment 2
[0025] Synthesis of Taxol-SA-PEG2000 (compound 2)
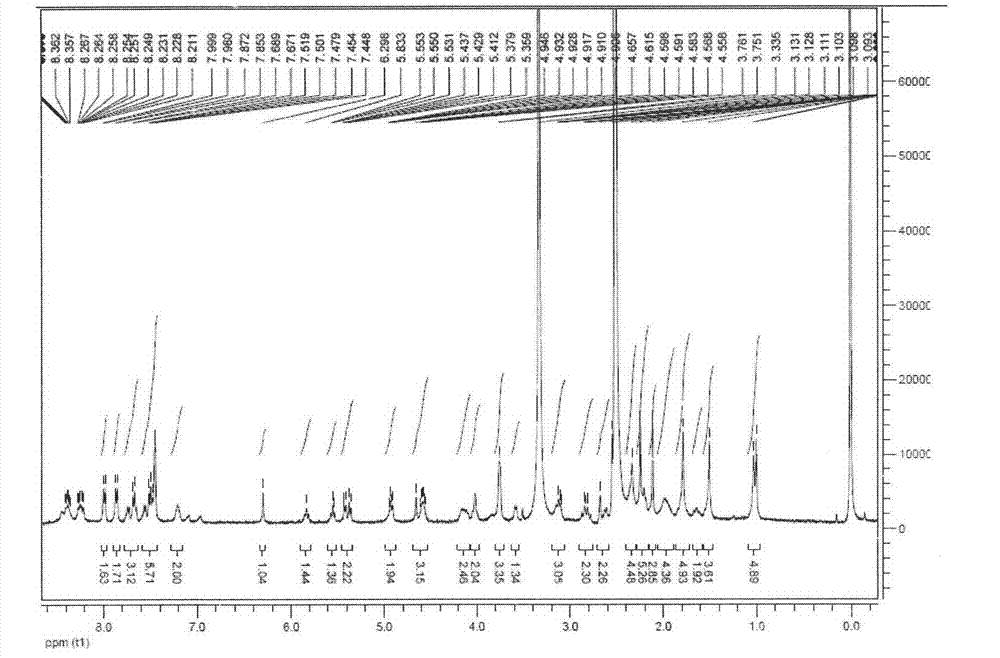
[0026]Weigh 200 mg (209.6 μmol) of Taxol-SA, add 10 mL of anhydrous dichloromethane (DCM), and add the following substances in sequence on an ice bath: 98.4 μL (628.8 μmol) of N, N′-dicyclohexylcarbodiimide (DIC ) and 10 mg DMAP, finally added 482 mg (241.04 μmol) of PEG2000, then returned to room temperature, stirred in the dark for 12 hours, spin-dried, washed once with 0.1N hydrochloric acid (10 mL), isopropanol crystallized, and the yield was 83.5%. 1 HNMR (300MHz, DMSO-d 6 ( m, 1H), 5.53(t, 1H), 5.31-5.41(m, 3H), 4.87-4.91(m, 2H), 4.54-4.63(m, 5H), 3.99-4.05(m, 6H), 3.71- 3.75(m, 4H), 3.43-3.65(s, PEG), 3.37-3.41(m, 8H), 3.22-3.28(m, 3H), 2.55-2.66(m, 3H), 2.23(s, 3H), 2.05-2.11(m, 3H), 1.75-1.77(m, 3H), 1.49(s, 3H), 1.09-1.15(m, 3H), 0.99-1.02(m, 6H).
[0027]
Embodiment 3
[0029] Synthesis of Taxol-PEG 600 (compound 3) (referring to literature J.Med.Chem.1996, 39, 424-431) Use a pipette to draw 120 μL of PEG 600 (polyethylene glycol dicarboxylic acid) carboxylated at both ends, As for the ice bath, add 10mL of dichloromethane, add 79uL N,N'-dicyclohexylcarbodiimide (DIC) and 10mg DMAP at the same time, then add 200mg paclitaxel, let it slowly return to room temperature, avoid light at room temperature Reacted for 12 hours, spin-dried, washed once with 0.1N hydrochloric acid (10 mL), freeze-dried, crystallized from isopropanol, and the yield was 80.2%. 1 HNMR (300MHz, CHCl 3 -d 6 )δ8.10-8.14 (m, 2H), 7.75-7.77 (m, 3H), 7.26-7.53 (m, 13H), 6.20-6.28 (m, 2H), 5.99-6.03 (m, 1H), 5.67- 5.70(d, 1H), 5.50-5.53(m, 1H), 4.95-4.98(d, 1H), 4.17-4.31(m, 7H), 4.07-4.12(m, 4H), 3.50-3.67(m, PEG ), 3.43-3.51(m, 5H), 2.47(s, 3H), 2.23(s, 2H), 2.14-2.17(m, 2H), 1.96(d, J=13.5, 3H), 1.80(s, 2H ), 1.68(s, 2H), 1.12-1.23(m, 11H)
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com