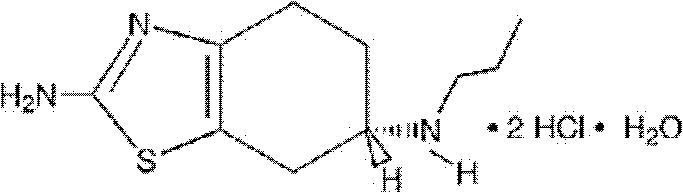
Preparation method for pramipexole preparation
A technology of pramipexole and preparations, applied in the field of pramipexole preparations and its preparation, can solve the problems of increased hygroscopicity and affecting the storage stability of finished preparations, and achieve low hygroscopicity, strong compressibility, and good storage stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
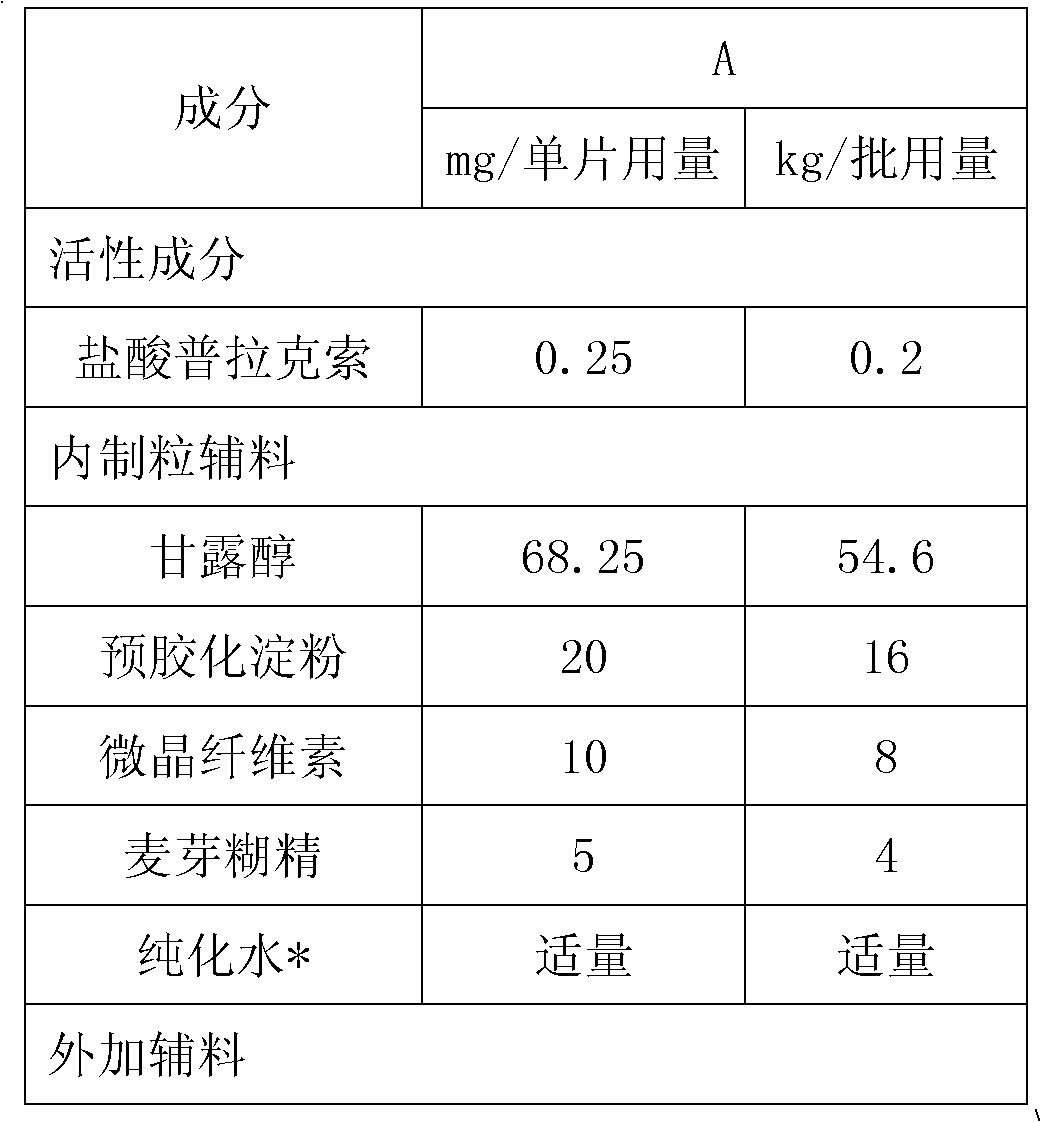
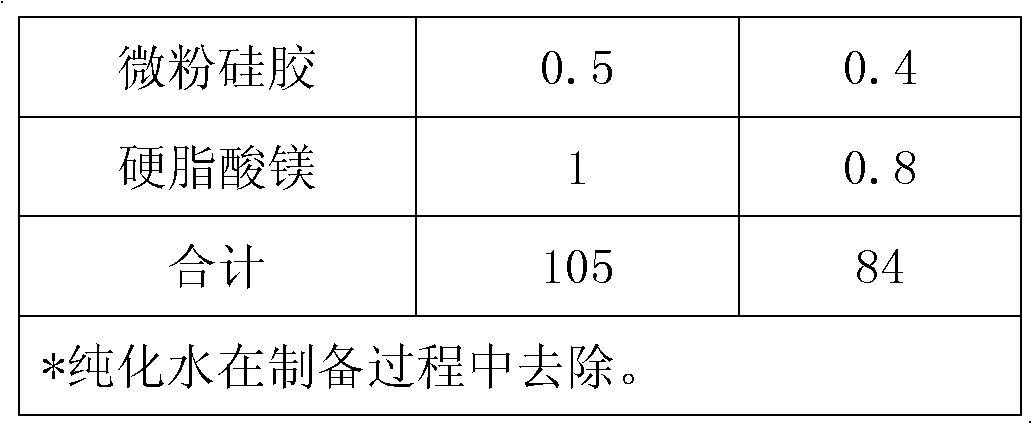
[0051] prescription:
[0052]
[0053]
[0054] Preparation: Weigh 4kg of maltodextrin into a stainless steel bucket, add 20kg of purified water, stir to dissolve; weigh 0.1kg of pramipexole hydrochloride in batches, add it to the prepared maltodextrin aqueous solution, and stir to obtain a clear solution; Pour batch amounts of mannitol, pregelatinized starch and microcrystalline cellulose into a high-speed wet granulator and mix them; then add pramipexole hydrochloride maltodextrin solution for granulation; the granules are transferred to a fluidized bed Drying, set the inlet air temperature to 70°C, take samples during the process, control the drying weight loss to be less than 2.0% (background temperature: 105°C); pass the dry granules through a 20-mesh sieve for granulation, then add additional silicon dioxide and magnesium stearate, mix Uniform; use 8*5mm ellipse flat slope punching plain tablet, theoretical tablet weight 102mg.
[0055] Sampling the intermediate, ...
Embodiment 2
[0059] prescription:
[0060]
[0061]
[0062] Note: Prescription B comes from the background technology WO2008122638 and does not belong to the present invention. It is used as a comparative example here to compare and investigate the moisture absorption of prescription C.
[0063] Place the tablets prepared by prescription B and prescription C at 25°C and 75% relative humidity, and measure the moisture absorption weight gain at each sampling point. The results are shown in Table 2:
[0064] Table 2 Investigation of moisture absorption
[0065]
[0066] The results of the moisture absorption test show that the sheet prepared according to the present invention has a lower degree of moisture absorption.
[0067] The pramipexole hydrochloride tablets of prescription B and prescription C were packaged in aluminum and plastic, and placed under the conditions of 60°C / 75% relative humidity and 40°C / 75% relative humidity for 4 weeks, and the content of the samples and rela...
Embodiment 3
[0080] prescription:
[0081]
[0082]
[0083] Preparation: Weigh the batch amount of pramipexole hydrochloride and add it to the prepared hydroxypropyl cellulose 50% ethanol solution, stir to dissolve; the batch amount of mannitol, pregelatinized starch and microcrystalline cellulose are granulated at high speed Mix in the machine; add hypromellose ethanol solution containing pramipexole hydrochloride for granulation; transfer the granules to a fluidized bed for drying, set the inlet air temperature at 60°C until the weight loss on drying is less than 2.0% (background temperature 105°C) ;Sizing the granules, then adding the extra micro-powdered silica gel and magnesium stearate, and mixing; adopting a φ6.5mm circular shallow concave punching plain tablet, the theoretical tablet weight is 100mg.
[0084] Vegetarian tablets at 0’HARA Coating on high-efficiency coating machine. Set the air inlet temperature to 60°C, and coat to a weight gain of 3.0%.
[0085] Sampling...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com