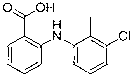
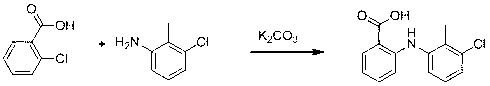Synthesis method of tolfenamic acid
A synthetic method, the technology of tolfenamic acid, applied in the field of drug synthesis, can solve the problems of cumbersome refining process, low product yield, and difficult operation, and achieve the effects of high yield, convenient purification treatment, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Step 1. Weigh 157g of o-chlorobenzoic acid and 40g of sodium hydroxide into 600mL of methyl isobutyl ketone, and heat to reflux for 0.5 h.
[0033] Step 2. Weigh 142 g of 3-chloro-2-methylaniline, 90 g of sodium acetate and 1 g of copper acetate into the reaction mixture obtained in the previous step, heat to reflux for 3 h, then cool, add 500 mL of water to extract , liquid separation, acidify the aqueous phase with concentrated hydrochloric acid to PH=2, continue to stir for 1h to crystallize, filter, the filter cake is the crude product of tolfenamic acid, and recrystallize the crude product of tolfenamic acid with ethanol / water mixed solvent to obtain tolfenamic acid Namic acid refined product 215g, yield 82%.
Embodiment 2
[0035] Step 1. Weigh 157g of o-chlorobenzoic acid and 40g of sodium hydroxide into 600mL of methyl isobutyl ketone, and heat to reflux for 0.5 h.
[0036] Step 2. Weigh 142 g of 3-chloro-2-methylaniline, 84 g of sodium bicarbonate and 1 g of copper powder and add them to the reaction mixture obtained in the previous step, heat to reflux for 4 h, then cool, and add 500 mL of water Extraction, liquid separation, acidify the aqueous phase with concentrated hydrochloric acid to PH = 2, continue to stir for 1h to crystallize, filter, the filter cake is the crude tolfenamic acid, recrystallize the crude tolfenamic acid with ethanol / water mixed solvent to obtain Fenamic acid refined product 209g, yield 80%.
Embodiment 3
[0038] Step 1. Weigh 157g of o-chlorobenzoic acid and 66g of potassium hydroxide into 600mL of methyl isobutyl ketone, and heat to reflux for 0.5 h.
[0039] Step 2. Weigh 156 g of 3-chloro-2-methylaniline, 118 g of potassium acetate and 1.5 g of cuprous bromide and add them to the reaction mixture obtained in the previous step reaction, heat and reflux for 3 h, then cool and add Extract with 500mL water, separate the liquids, acidify the aqueous phase with concentrated hydrochloric acid to PH=2, continue to stir for 1h to crystallize, filter, the filter cake is the crude tolfenamic acid, recrystallize the crude tolfenamic acid with methanol to obtain tolfenamic acid Acid refined product 191g, yield 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com