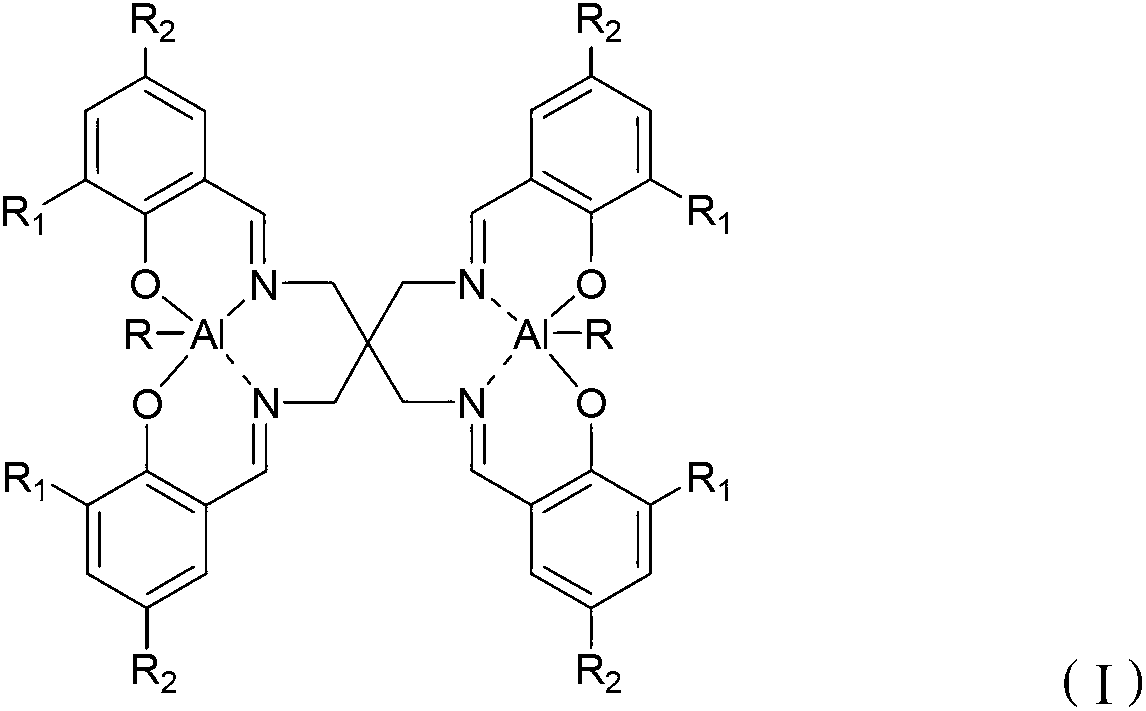
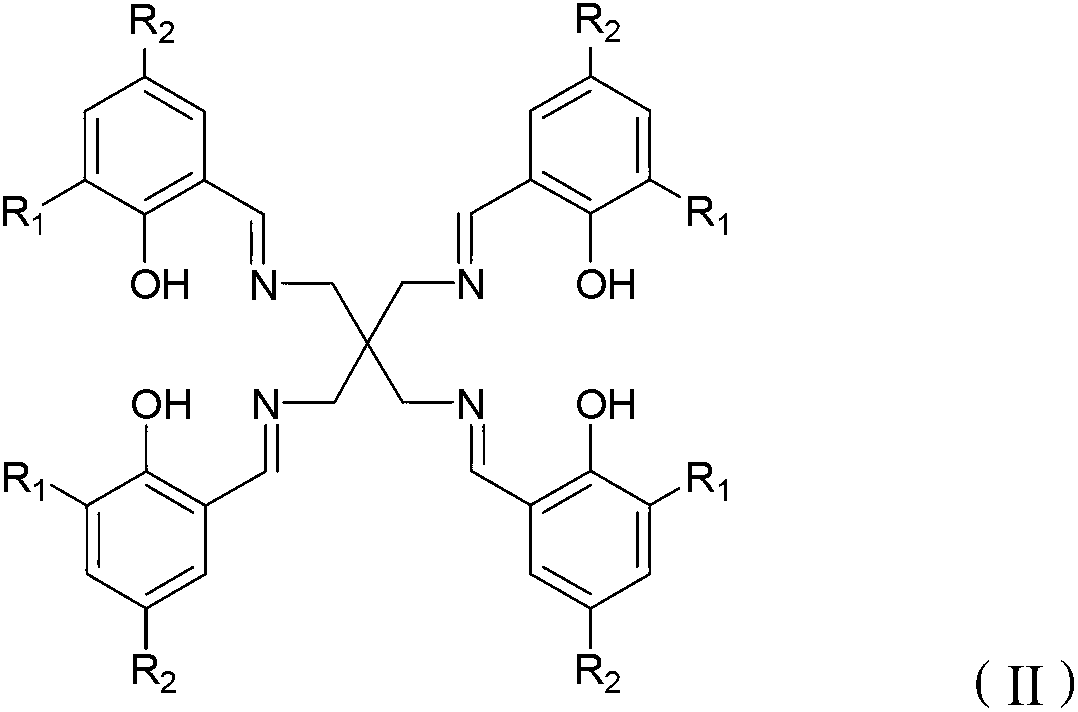
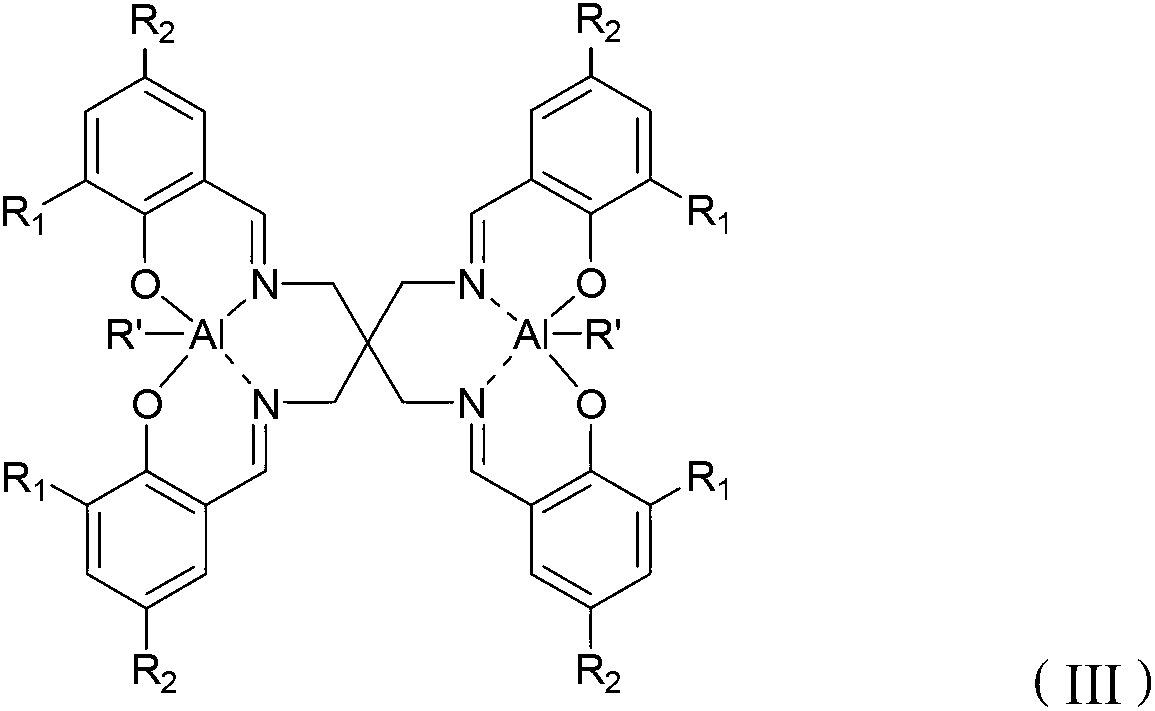
Schiff base aluminum compound, preparation method thereof and preparation method of polylactic acid
A technology of Schiff base aluminum and compound, which is applied in the preparation of polylactic acid, Schiff base aluminum compound and its preparation field, can solve the problems of catalytic reaction selectivity and low activity, achieve selectivity enhancement, enhance reaction activity, improve active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] Optionally, when R is -CH 3 or -CH 2 CH 3 , the present invention also provides a method for preparing a Schiff base aluminum compound, comprising the following steps: combining the Schiff base with the structure of formula (II) and Al(R′) 3 React in a solvent to obtain the Schiff base aluminum compound with the structure of formula (III). Wherein, the solvent is an organic solvent well known to those skilled in the art, preferably tetrahydrofuran or toluene.
[0049] R 1 and R 2 The choice of influences the choice of solvent, when R 1 and R 2 independently selected from -H, -F, -Cl, -Br or -NO 2 When, the reaction solvent is preferably tetrahydrofuran, when R 1 and R 2 independently selected from -CH 3 、-CH 2 CH 3 、-CH(CH 3 ) 2 、-C(CH 3 ) 3 When, the reaction solvent is preferably toluene.
[0050]
[0051] R 1 and R 2 independently selected from -H, -CH 3 、-CH 2 CH 3 、-CH(CH 3 ) 2 、-C(CH 3 ) 3 , -F, -Cl, -Br or -NO 2 ;
[0052] R' is -CH...
Embodiment 1
[0074] The preparation of embodiment 1 pentaerythramine
[0075] 1.1 Stir 13.6g of pentaerythritol and 100ml of pyridine in an ice bath, slowly add 51.5g of methanesulfonyl chloride, and react for 3 hours, pour the reaction solution into a mixture of 200ml of concentrated hydrochloric acid, 400ml of water and 1000ml of methanol, filter, and separate After washing with water and methanol three times, it was sucked dry to obtain compound VIa.
[0076]1.2 Slowly heat 11.2g of the compound VIa obtained in 1.1, 500ml of DMSO and 10g of sodium azide to 100°C while stirring, and after 25 hours of reaction, pour the reaction system into 600ml of water, extract three times with petroleum ether, and combine the organic phases , washed three times with water, dried over anhydrous magnesium sulfate, filtered, and the organic solvent was removed by rotary evaporation to obtain white crystals as compound VIb.
[0077] 1.3 Under the conditions of ice bath and argon protection, add 1.5g of l...
Embodiment 2
[0078] Embodiment 2 structural formula is the synthesis of the Schiff base IIa of II
[0079] IIa: R 1 =R 2 =-H
[0080] Dissolve 1.32g of the pentaerythramine obtained in 1.3 in 20ml of ethanol, slowly dropwise add 50ml of ethanol containing 4.88g of salicylaldehyde, reflux for 14h, remove most of the solvent by rotary evaporation, filter to obtain a yellow powder, mix with chloroform and ethanol Solvent washing affords the Schiff base IIa.
[0081] Utilize nuclear magnetic resonance to analyze the Schiff base IIa obtained in embodiment 2, obtain its hydrogen spectrum, the result is as follows:
[0082] 1 H NMR (300.00MHz, d 6 -DMSO): δ=13.24(s, OH 4H), 8.61(s, NCH 4H), 7.45, 6.86(m, PhH 16H), 3.76(s, CCH 2 N 8H).
[0083] The Schiff base IIa obtained in Example 2 was analyzed by elemental analysis to obtain the content of each atom.
[0084] Elem. Anal. (%): Calcd. C 72.24; H 5.88; N 10.21. Found: C 72.13; H 5.81; N 10.20.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com