Method for detecting seven aromatic amine compounds in human urine through liquid chromatography-series mass spectrometry
A technology of tandem mass spectrometry and liquid chromatography, which is applied in the field of detection of seven aromatic amine compounds in human urine by liquid chromatography-tandem mass spectrometry, can solve cumbersome and time-consuming problems, and achieve simplified sample pretreatment steps, high sensitivity, and improved Analyzing the Effects on Efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
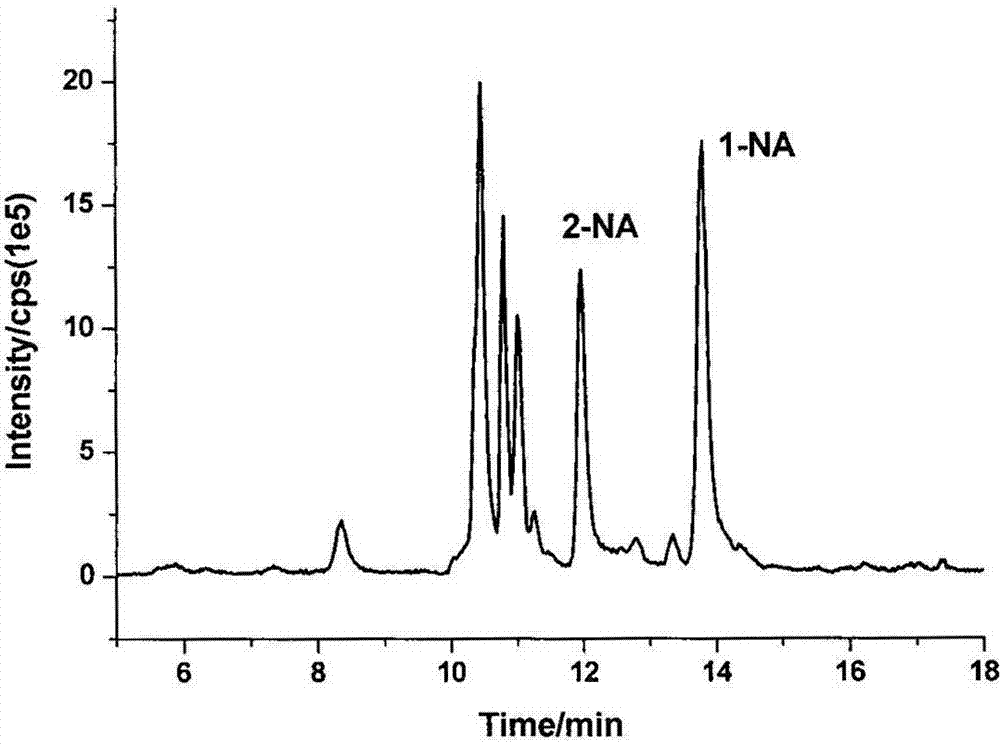
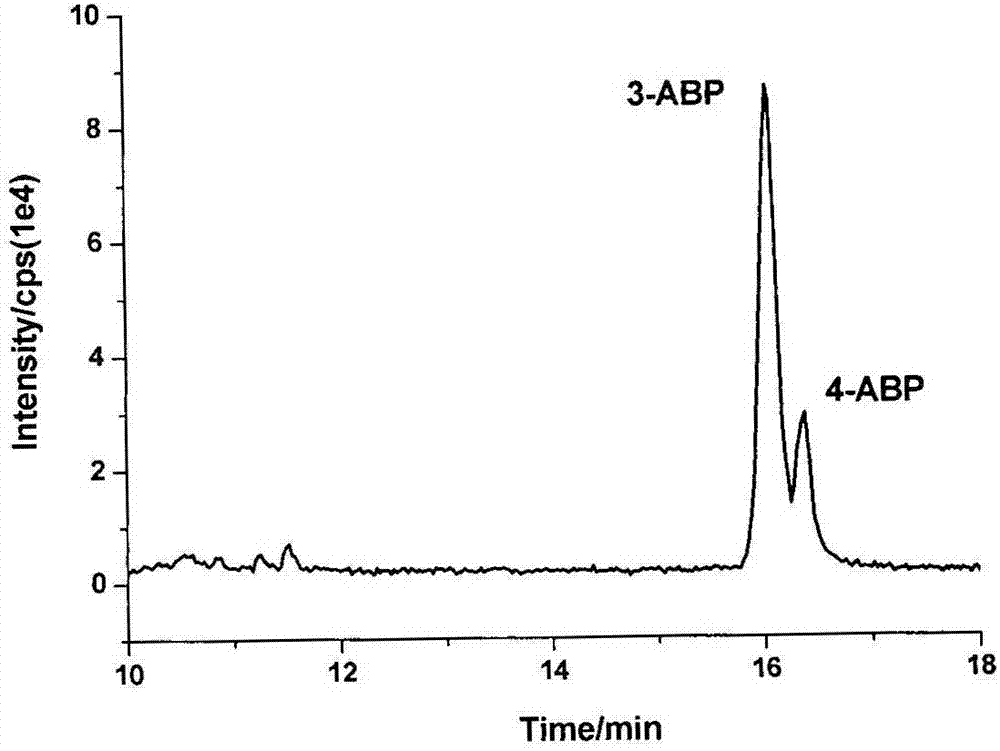
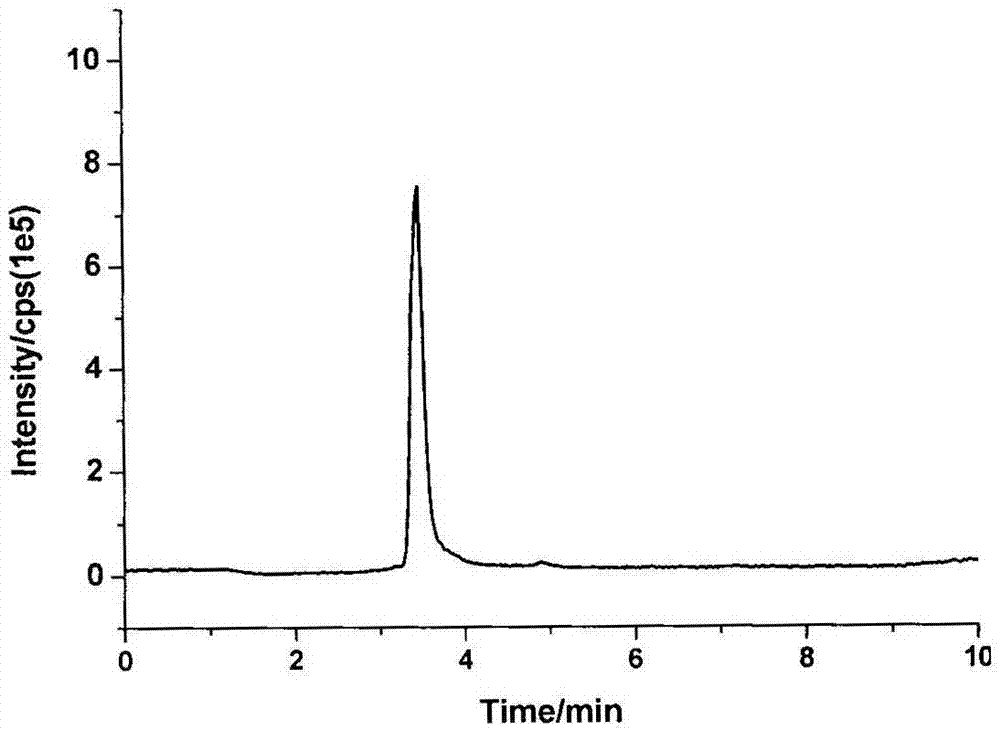
[0038] will be -18 o Urine samples stored in C were removed and thawed at room temperature. Take 5 mL of the thawed urine sample and place it in a 50 mL plastic centrifuge tube, add 1 mL of concentrated hydrochloric acid solution, 80 oC water bath for 1 h. After the sample was cooled, about 1.2 mL of 10 mol / L NaOH solution was added (to neutralize the sample solution to a pH of about 7.0), followed by 10 mL of ammonium acetate-ammonia buffer solution (0.5 mol / L, pH 7.0). Add 50 μL D7-1-aminonaphthalene and D5-aniline mixed internal standard solution. The samples were purified using a PAH solid-phase extraction column (Supel MIP& reg; pk of 50, 3 mL) produced by Shanghai Anpu Company. The sample solution was loaded onto a PAH molecular imprinting column activated with 1 mL cyclohexane, washed with 1 mL cyclohexane, and finally eluted with 10 mL ethyl acetate. The eluate was blown dry with nitrogen, reconstituted by adding 100 μL of methanol solution, and analyzed by LC-MS / M...
Embodiment 2
[0040] Randomly select 10 urine samples, take 100 mL each after thawing at room temperature and mix well. Take 5 mL of mixed urine sample, perform sample pretreatment according to the steps described in Example 1, and perform LC-MS / MS analysis. Five parallel experiments were carried out on the same day, and the relative standard deviation of each compound was between 5.36% and 7.87%, indicating that the method has good reproducibility and stability.
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com