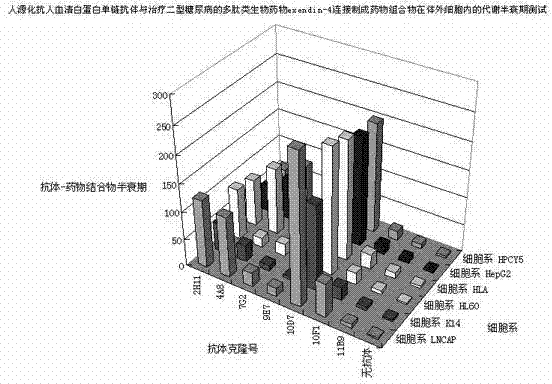
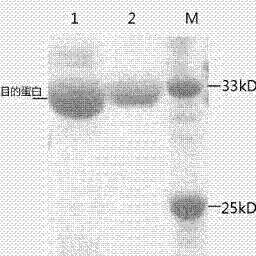
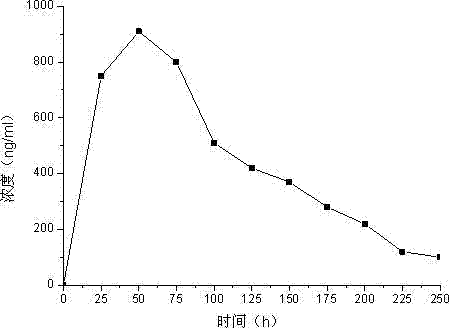
Anti-human serum albumin single-chain antibody and method for connecting polypeptide drugs to carbon end thereof
A single-chain antibody and drug technology, applied in the direction of anti-animal/human immunoglobulin, drug combination, pharmaceutical formula, etc., to achieve the effects of easy large-scale production, avoiding immunogenicity, and weak immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1, obtaining the single-chain antibody in the present invention:
[0030] 1. Construction of human antibody library
[0031] Screen the variable region sequences of antibodies with specific recognition ability for human serum albumin from the humanized single chain antibody library. The gene fragment is amplified by polymerase chain reaction and spliced into a phage vector. The primers are as follows. An example of primers used to find the light chain CDR: Forward primer [5'-GATATCCAACTTACCCAATCC-3'] Reverse primer [5'-CCTCTTTATTTCTACCTTGG-3'], an example of primers for searching heavy chain CDR: forward primer [5'-GAAGTCCAGCTGCTCG-3'], reverse primer [5'-CGAAGAGACTGTGACTAGCGT-3'].
[0032] Antibody molecular fragments are expressed by phage and displayed on the surface of phage, and human serum albumin (HSA) is used as an antigen to screen the antibodies displayed on the surface of phage to obtain the antibody with the best specificity.
[0033] 2. Antibody...
Embodiment 2
[0039] Example 2, Analysis of the coding sequence of the single-chain antibody, construction of recombinant and expression vectors:
[0040]Propagate the 10D7 strain produced in Example 1 in LB culture medium, then use a DNA extraction kit to purify the plasmid in the cell line, and separate it by restriction endonuclease cutting and 2% agarose gel electrophoresis to obtain The coding sequence of the variable region of the heavy chain, the coding sequence of the variable region of the light chain was obtained by the same method, and the human antibody variable region universal primers were used for PCR amplification, and then the obtained amplification products were sent to Eurofin for sequencing. The results of the obtained DNA sequence and protein sequence are shown in Table 2.1 and Table 2.2, wherein the sequence listed in Table 2.1 is the light chain region (C-SEQ-04) of the humanized anti-human serum albumin single chain antibody, and Its variable region sequence (C-SEQ-0...
Embodiment 3
[0044] Example 3, linking the light and heavy chain sequences of the single-chain antibody and exendin-4, a polypeptide biological drug for the treatment of type 2 diabetes, to form a drug combination:
[0045] The DNA of the light chain region and the heavy chain region of the antibody whose sequence has been determined is linked by direct synthesis or recombinant PCR. The hinge part is the VH chain region of the single-chain antibody with the DNA sequence corresponding to SEQ ID No.2. The operation method of PCR is a conventional method commonly used in the field. The sequencing of the sequence synthesized by Invitrogen Company is completely the same as the sequencing result of the link sequence of the antibody light and heavy chains completed by the method of recombinant PCR. The sequencing results are shown in the table 3.1 shows:
[0046] GAT ATC CAA CTT ACC CAA TCC CCA AGT AGC [V L ] 5 10 CTT TCG GCC TCG ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com