Biomass-based phenolic resin and preparation method thereof
A technology based on phenolic resin and phenolic resin, applied in the field of new phenolic resin polymer materials, can solve the problems of limited reserves, serious pollution, non-renewable, etc., and achieve the effect of avoiding oxidation and avoiding the polymerization process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
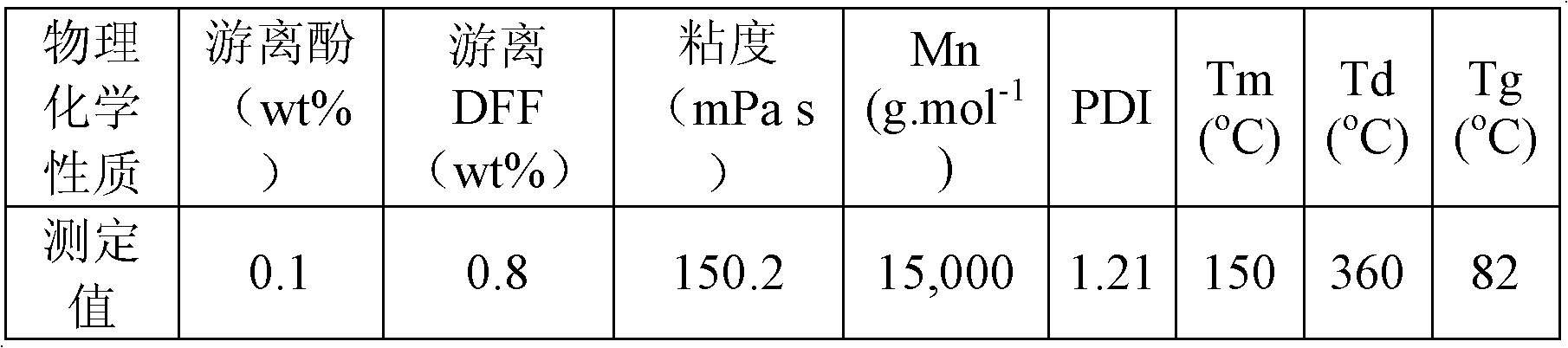
[0023] Example 1: 50 mL of an aqueous solution of 10 mmol 2,5-diformylfuran, 50 mL of an aqueous solution of 5 mmol phenol and 0.5 mmol sodium hydroxide were simultaneously added dropwise into a 250 mL four-neck round bottom flask. Under the protection of nitrogen, react at 40°C for 0.5h; then raise the temperature to 120°C, and react at this temperature for 3h. Water and other impurities in the reaction system were then distilled off under reduced pressure. After cooling to room temperature, the obtained polymer was a milky white solid, which was vacuum-dried at 50°C for 12 hours. The molar yield of the polymer is 90% based on the feed amount of the monomer 2,5-diformylfuran. NMR spectrogram measurement results and attribution analysis are: 1 H NMR (TFA-d 1 , ppm): 6.50-7.00 (m, benzene-H), 5.76 (s, furan-H), 5.57 (s, -CH-), 5.0 (s, -OH); 13 C NMR (TFA-d 1 , ppm): 36.4 (-CH-), 107.4 (C 3 / C 4 ), 110-160 (benzene-C). Infrared spectrogram measurement results and attribu...
Embodiment 2
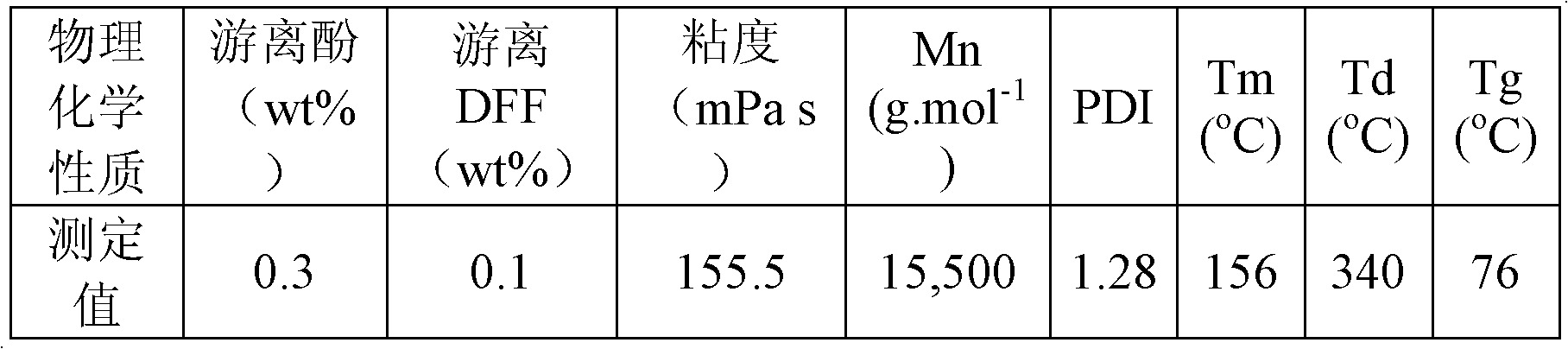
[0026] Example 2: 50 mL of an aqueous solution of 10 mmol 2,5-diformylfuran, 50 mL of an aqueous solution of 40 mmol p-cresol and 0.05 mmol sodium carbonate were simultaneously added dropwise to a 250 mL four-neck round bottom flask. Under the protection of nitrogen, react at 50°C for 1h; then raise the temperature to 100°C, and react at this temperature for 4h. Water and other impurities in the reaction system were then distilled off under reduced pressure. After cooling to room temperature, the obtained polymer was a milky white solid, which was vacuum-dried at 50°C for 12 hours. Calculated according to the feeding amount of monomer 2,5-diformylfuran, the molar yield of the polymer is 95%. NMR spectrogram measurement results and attribution analysis are: 1 H NMR (TFA-d 1 , ppm): 6.50-7.00 (m, benzene-H), 5.76 (s, furan-H), 5.57 (s, -CH-), 5.0 (s, -OH), 2.35 (s, -CH3). 13 C NMR (TFA-d 1 , ppm): 24.3 (-CH 3 ), 36.4(-CH-), 107.4(C 3 / C 4 ), 110-160 (benzene-C). Infrare...
Embodiment 3
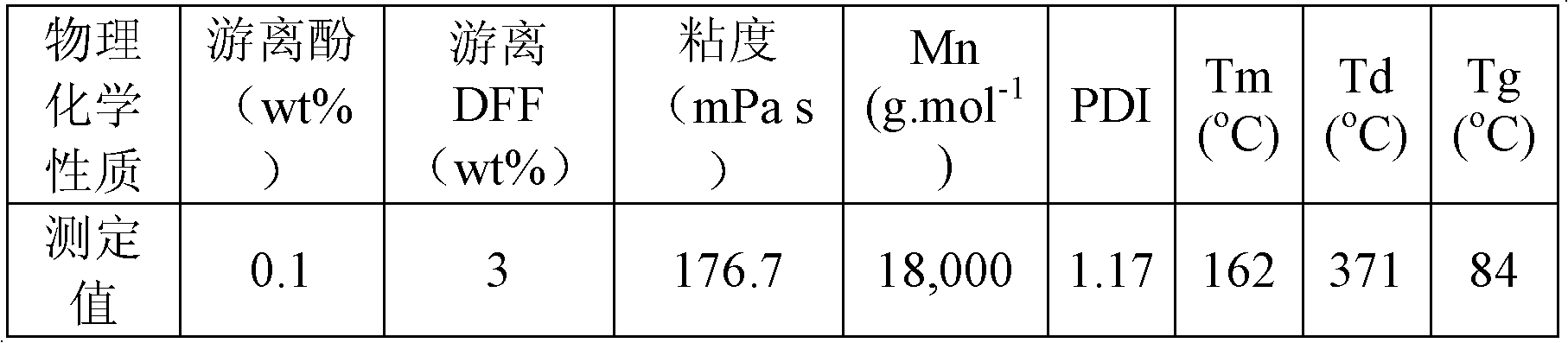
[0029] Example 3: 50 mL of an aqueous solution of 10 mmol 2,5-diformylfuran, 50 mL of an aqueous solution of 100 mmol p-tert-butylphenol and 1 mmol potassium hydroxide were simultaneously added dropwise to a 250 mL four-neck round bottom flask. Under the protection of nitrogen, react at 60°C for 5h; then raise the temperature to 180°C, and react at this temperature for 0.5h. Water and other impurities in the reaction system were then distilled off under reduced pressure. After cooling to room temperature, the obtained polymer was a milky white solid, which was vacuum-dried at 50°C for 12 hours. The molar yield of the polymer is 96% based on the feed amount of the monomer 2,5-diformylfuran. NMR spectrogram measurement results and attribution analysis are: 1 H NMR (TFA-d 1 , ppm): 6.50-7.00 (m, benzene-H), 5.76 (s, furan-H), 5.57 (s, -CH-), 5.0 (s, -OH), 1.34 (s, -C (CH 3 ) 3 -). 13 C NMR (TFA-d 1 , ppm): 36.4(-CH-), 31.4(-C(CH 3 ) 3 -), 40.7(-C(CH 3 ) 3 -), 107.4(C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com