Aripiprazole preparation and preparation method thereof
A technology for aripiprazole and preparations, applied in the field of aripiprazole preparations and its preparation, capable of solving problems such as unsatisfactory stability of aripiprazole preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
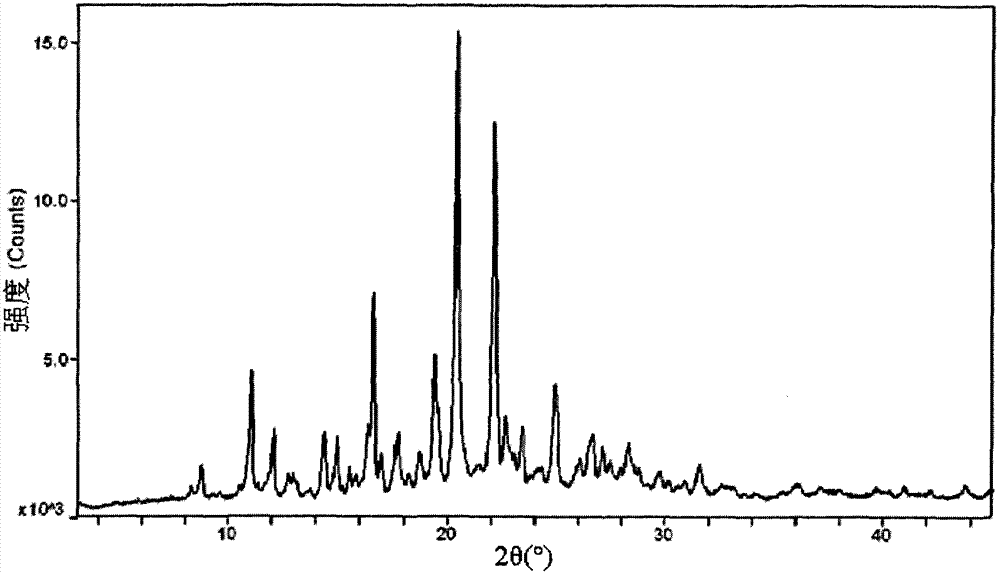
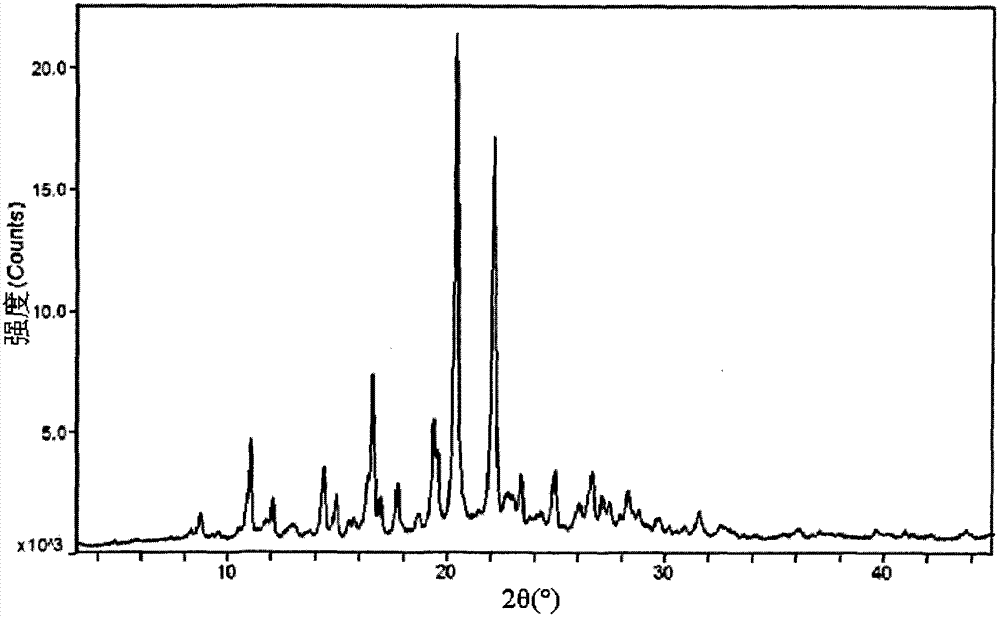
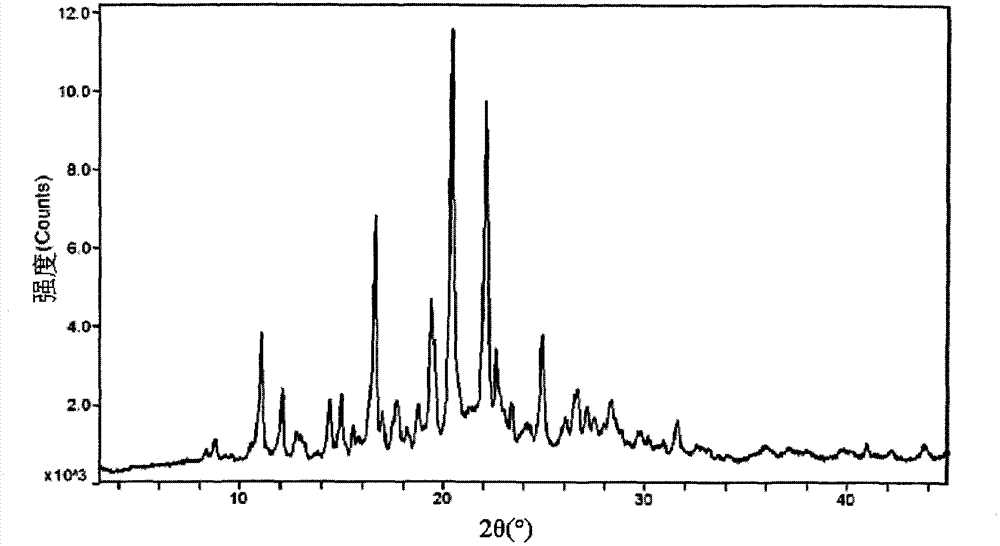
Image
Examples
Embodiment 1
[0060] Comparative example 1 Aripiprazole tablet (10mg / tablet) formula and preparation method (unit: gram)
[0061]
[0062] (10mg / tablet) formula and preparation method of embodiment 1 aripiprazole tablet (unit: gram)
[0063]
Embodiment 2
[0064] Embodiment 2 Aripiprazole Capsules (10mg / grain) formula and preparation method
[0065] The granules (including sodium starch glycolate and magnesium stearate) before the tablet compression of Example 1 were passed through a 30-mesh sieve and mixed homogeneously, and packed into capsules.
Embodiment 3
[0066] Embodiment 3 Formula and preparation method of aripiprazole tablet (20mg / tablet) (unit: gram)
[0067]
[0068]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com