Synthesis method of menthalactone
A synthesis method and a technology for mentholide, applied in directions such as organic chemistry, can solve problems such as unsuitable large-scale preparation and industrial production, harsh reaction conditions, and many synthesis steps, and achieve mild reaction conditions, good yield, and simplified preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
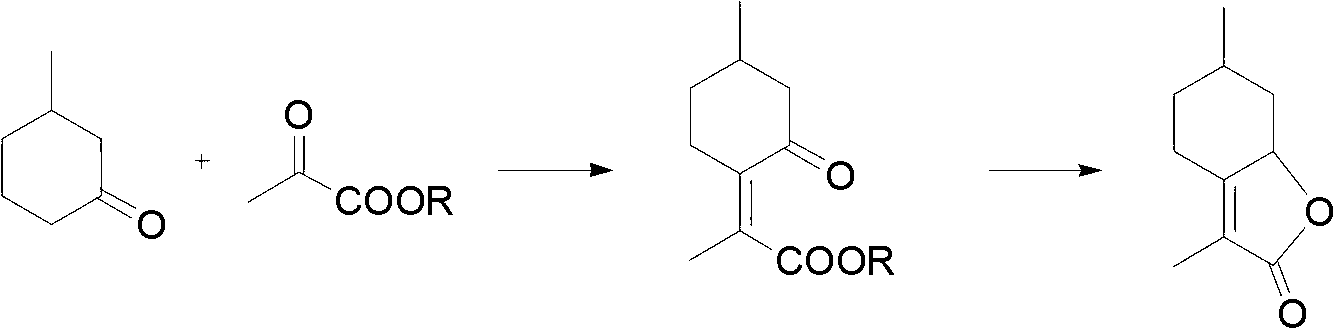
[0024] 2.0 g of 3-methylcyclohexanone, 3.0 g of pyruvate and 2.0 g of anhydrous ferric chloride powder were mixed in a 100 mL single-necked bottle, and stirred and reacted at 90° C. for 6 h. GC-MS detection reaction progress. After the reaction was completed, 70 mL of dichloromethane was added to the reaction mixture, the solid salt was filtered off with suction, the dichloromethane layer was washed with saturated brine, the solvent was evaporated under reduced pressure, and 10% sodium hydroxide solution was added at 50°C. Saponify for 3 hours, then cool the water bath temperature to 5°C, add 0.4 g of sodium borohydride solid in batches, wait for it to rise to room temperature naturally, stir for a total of 5 hours, acidify the solution with 20% sulfuric acid to pH = 1, and extract water with dichloromethane layer, concentrated, and purified by column chromatography to obtain 1.70 g of mentholactone with a yield of 57%. The spectral data of mentholactone is: 1 H NMR:δ0.91-1....
Embodiment 2
[0026] 2.0 g of 3-methylcyclohexanone, 1.0 g of pyruvate and 1.5 g of anhydrous copper chloride were mixed in a 100 mL single-necked bottle, and stirred and reacted at 95° C. for 4 h. GC-MS detection reaction progress. After the reaction is complete, add 70 mL of dichloromethane to the reaction mixture, filter off the solid salt, wash the dichloromethane layer with saturated brine, evaporate the solvent under reduced pressure, add 10% sodium hydroxide solution and saponify at 50°C After 3 hours, cool the temperature of the water bath to 5°C, add 0.4 g of solid sodium borohydride in batches, wait for it to rise to room temperature naturally, stir for a total of 5 hours, acidify the solution with 20% sulfuric acid to pH = 1, and extract the water layer with dichloromethane , concentrated, and purified by column chromatography to obtain 0.82 g of mentholactone, with a yield of 55%.
Embodiment 3
[0028] 2.0 g of 3-methylcyclohexanone, 3.0 g of pyruvate and 2.0 g of anhydrous aluminum trichloride powder were mixed in a 100 mL single-necked bottle, and stirred and reacted at 90° C. for 6 h. GC-MS detection reaction progress. After the reaction was completed, 70 mL of dichloromethane was added to the reaction mixture, the solid salt was filtered off with suction, the dichloromethane layer was washed with saturated brine, the solvent was evaporated under reduced pressure, and 10% sodium hydroxide solution was added at 50°C. Saponify for 3 hours, then cool the water bath temperature to 5°C, add 0.4 g of sodium borohydride solid in batches, wait for it to rise to room temperature naturally, stir for a total of 5 hours, acidify the solution with 20% sulfuric acid to pH = 1, and extract water with dichloromethane layer, concentrated, and purified by column chromatography to obtain 0.36 g of mentholactone, with a yield of 12%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com