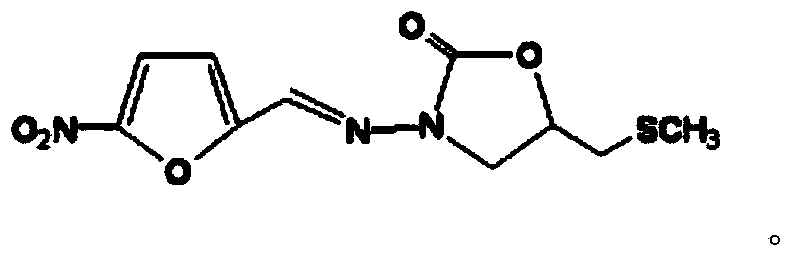
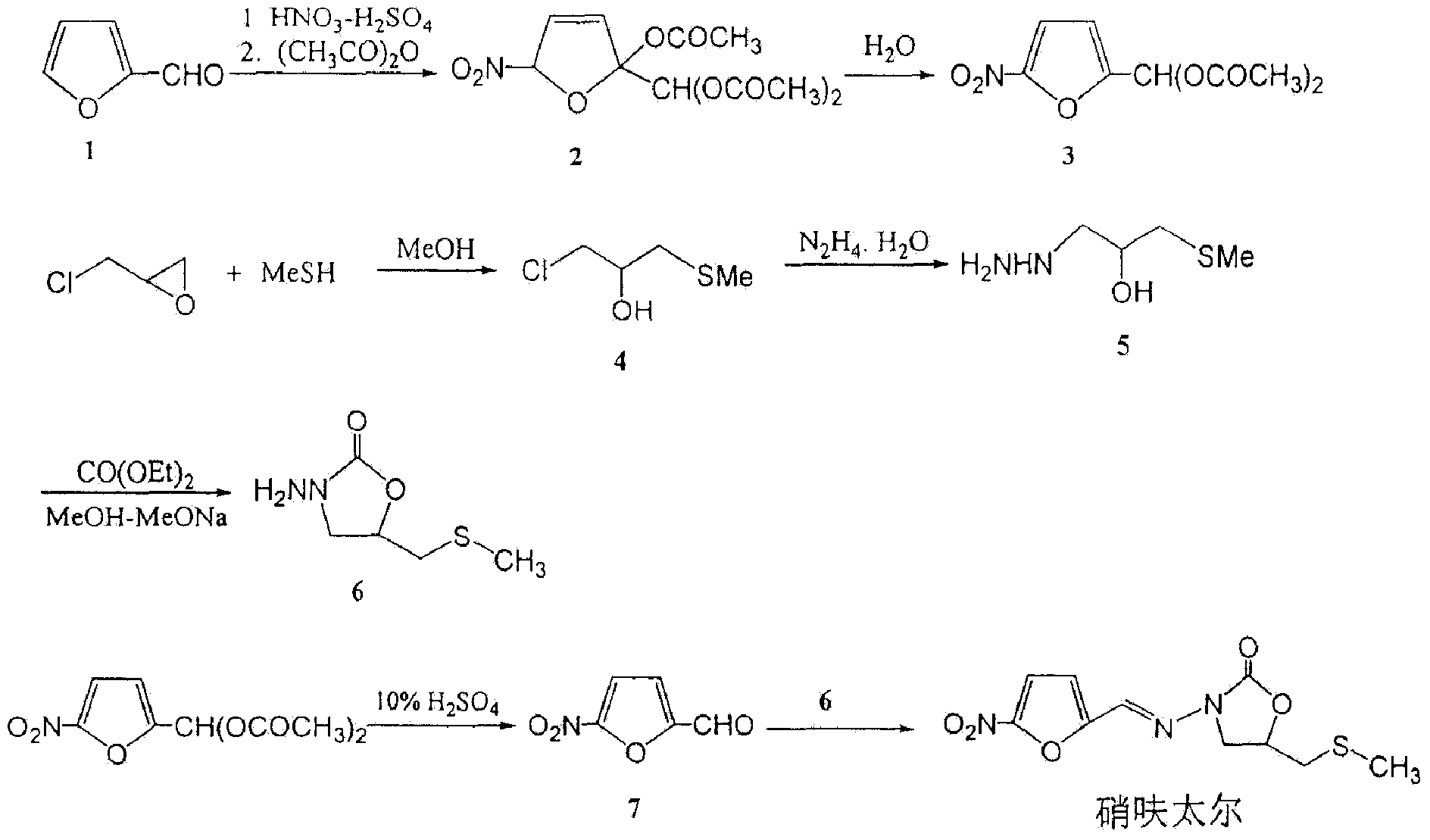
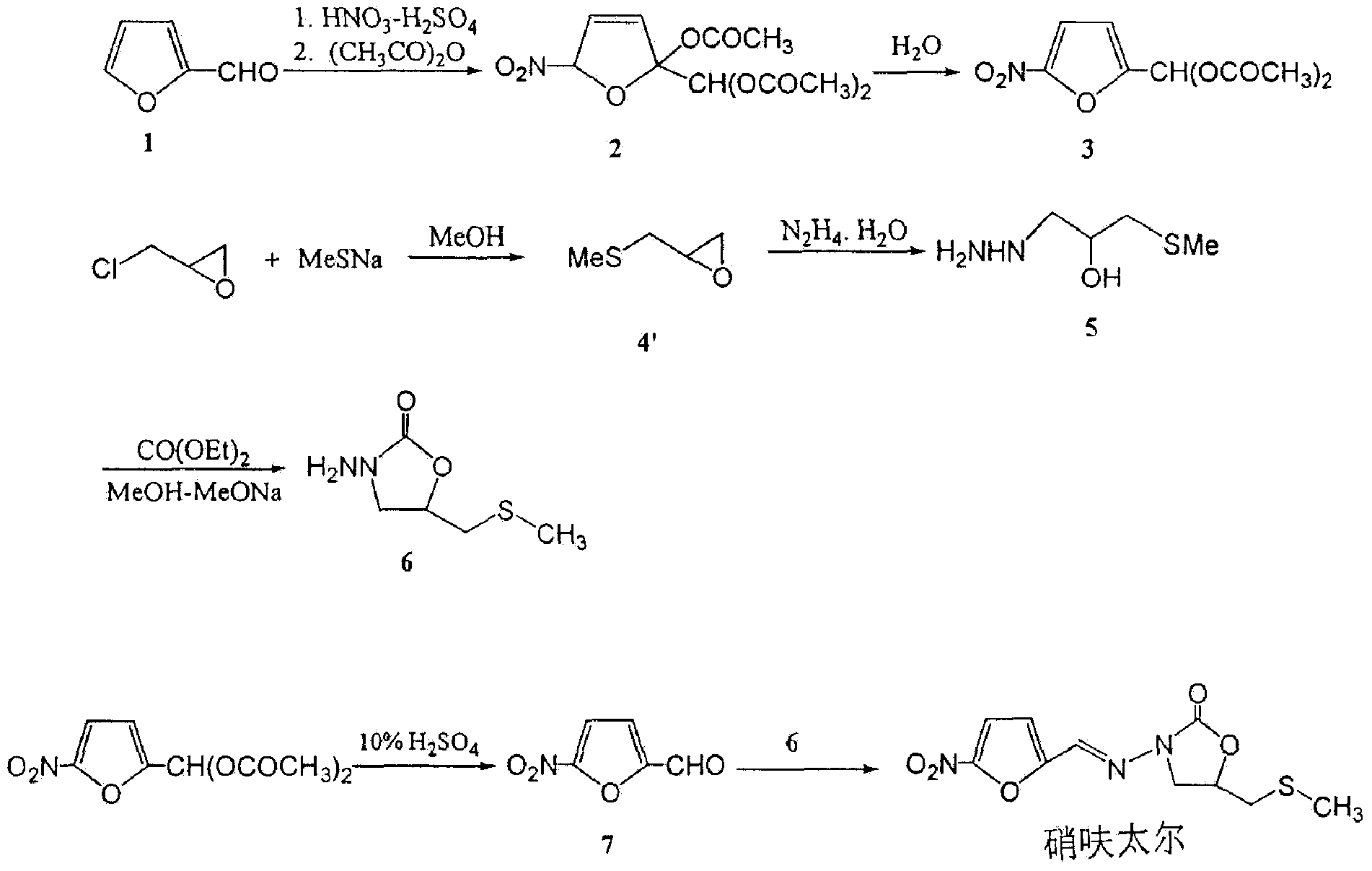
Synthetic method of nifuratel
A synthetic method, the technology of nifuratel, applied in the field of drug synthesis, can solve the problems of low reaction rate and yield, many impurities in the product, explosion hazard, etc., and achieve the effect of easy control of the process, easy availability of raw materials, and reduction of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the process condition optimization of the synthetic method step 1 of nifuratel
[0029] During the synthesis process of nifuratel, the present invention investigates the effect of the temperature of the substitution reaction described in step 1 of the synthesis method of nifuratel on the color of the reaction solution and the yield of 2-(methylthiomethyl)-oxirane The results are shown in Table 1.
[0030] Table 1 Process conditions of step 1
[0031] temperature reflex
[0032] As can be seen from the results in Table 1, in the synthetic process of nifuratel, the higher the substitution reaction temperature described in step 1, the more yellow impurities generated, and the yield of intermediate 2-(methylthiomethyl)-oxirane lower. Therefore, the reaction temperature is determined to be 0-10°C. Further detection of the reaction product between the residual liquid and sodium methylmercaptide after distillation showed that no 2-(methylthiomethyl)...
Embodiment 2
[0033] Embodiment 2: the process condition optimization of the synthetic method step 2 of nifuratel
[0034] During the synthesis of nifuratel, the post-treatment method of the intermediate 3-methylthio-2-hydroxyl-propylhydrazine further prepared by the present invention was studied, and the results are shown in Table 2.
[0035] Table 2 The post-treatment process conditions of 3-methylthio-2-hydroxyl-propylhydrazine
[0036]
[0037] As can be seen from the results in Table 2, in the synthetic process of nifuratel, if the reaction solution is not subjected to high vacuum distillation, the quality of the final nifuratel product will be more affected. Therefore, it is determined that the reaction solution is first evaporated to remove hydrazine hydrate and water at a lower temperature and vacuum, and then distills high-purity 3-methylthio-2-hydroxyl-propylhydrazine under high vacuum.
Embodiment 4
[0038] Embodiment 4: the process condition optimization of the synthetic method step 3 of nifuratel
[0039] During the synthesis process of nifuratel, the present invention investigated the influence of different hydrolysis solvents on the preparation of intermediate 5-nitrofurfural and the final product, and the results are shown in Table 3.
[0040] The influence of table 3 different solvents on the preparation of intermediate 5-nitrofurfural
[0041] Hydrolysis solvent
[0042] As can be seen from the results in Table 3, compared with dilute hydrochloric acid, 5-nitrofurfural diethyl ester was refluxed in dilute sulfuric acid and hydrolyzed for 30 minutes to easily obtain 5-nitrofurfural solution, and the final nifuratel product quality was also qualified Therefore, it is determined to use the process of hydrolyzing 5-nitrofurfural diethyl ester in dilute sulfuric acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com