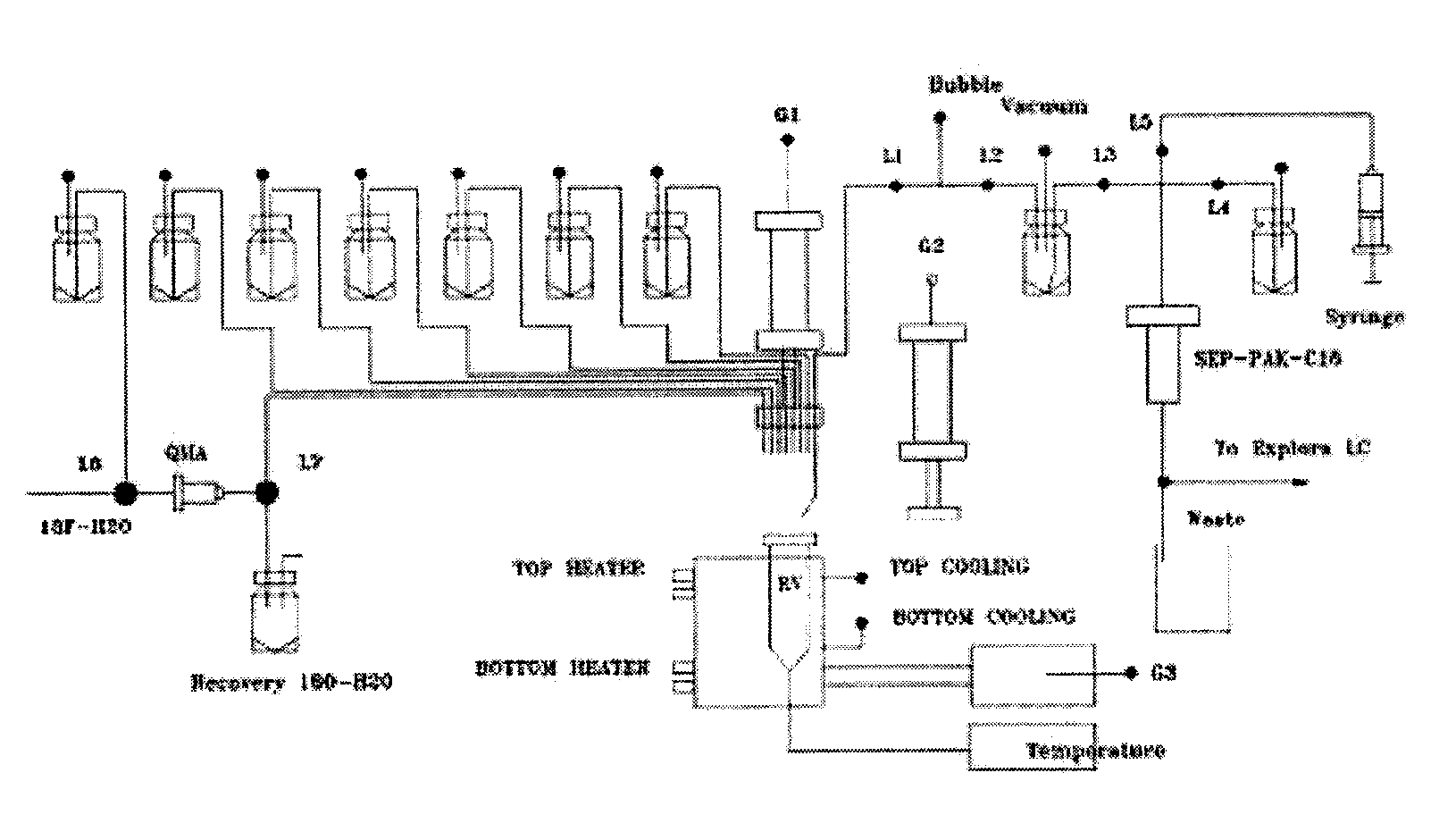
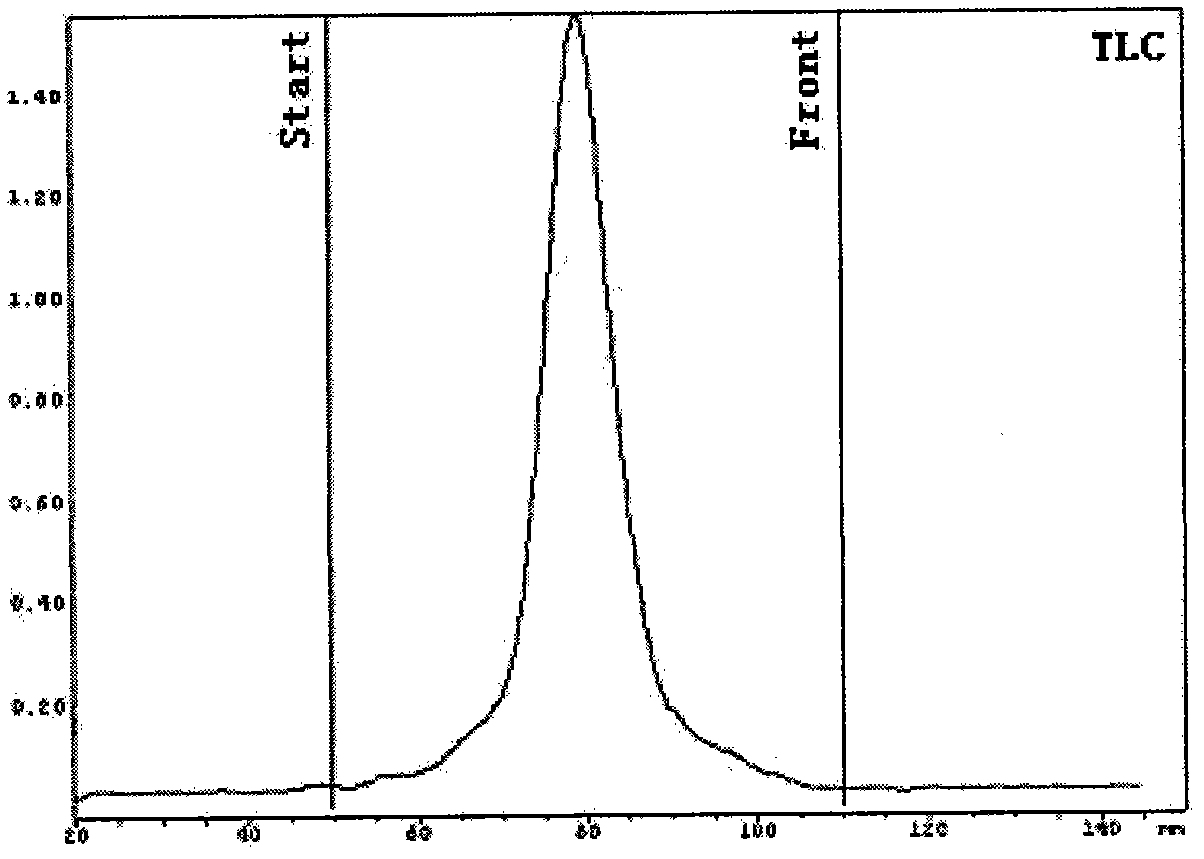
Automated synthesizer of <18>F-marked PET (positron emission tomograph)/CT (computerized tomograph) molecular image probe
A molecular imaging and synthesizer technology, applied in the fields of nuclear medicine and molecular imaging, can solve the problems of complex design, high production cost, lack of generality and flexibility, etc., and achieve the effect of stable performance and long service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: 18 Automated synthesis of F-FDG
[0034] 18 f - production: using 18 O(p,n) 18 For nuclear reactions, apply a volume of 2.4 ml of HO 2 O[ 18 O] Oxygen-enriched water (95%) target is continuously bombarded with a proton beam of 11 MeV and 35 μA for 30 to 60 min on the cyclotron to obtain the [ 18 F] fluoride ion oxygen-enriched aqueous solution.
[0035] Start this automated synthesizer and write automated synthesis 18 F-FDG prescription program, and to run the program, complete all of the following steps:
[0036] 18 F-separation: the 18 F-Oxygen-enriched aqueous solution is passed through the QMA column, and the 18 F ions are enriched on the QMA column, while oxygen-enriched water is collected in the recovery bottle. Transfer 1ml K 222 / K 2 CO 3 Acetonitrile / water solution (K 222 , 15mg / ml; K 2 CO 3 , 1.2mg / ml) through the QMA column, eluting 18 F ions and collect them in the reaction vial.
[0037] 18 f - Drying / activation of the react...
Embodiment 2
[0041] Example 2: 18 Automated synthesis of F-FLT
[0042] 18 f - Production: ditto.
[0043] Start this automated synthesizer and write automated synthesis 18 F-FLT's prescription program, and to run the program, complete all of the following steps:
[0044] 18 f - Separation: Same as above.
[0045] 18 f - Drying / Activation: Same as above.
[0046] 18 F labeling reaction: transfer 1 ml of the precursor 3-N-t-butoxycarbonyl-5′-O-dimethoxytriphenyl-3′-O-p-nitrobenzenesulfonyl-thymine in anhydrous acetonitrile ( 30mg / ml) into the reaction vial, heated at 100°C, and sealed for 10 minutes.
[0047] Hydrolysis reaction: transfer 1.0ml of 1M HCl solution to the reaction bottle, heat at 100°C, seal the reaction for 5min; then transfer and add 1.5ml of 2M NaOAc solution for neutralization.
[0048] Separation and purification: transfer the neutralized solution through Al 2 o 3 Column, injected into the sample loop of semi-preparative HPLC, run HPLC, the separation colu...
Embodiment 3
[0049] Example 3: 18 Automated synthesis of F-FES
[0050] 18 f - Production: ditto.
[0051] Start this automated synthesizer and write automated synthesis 18 F-FES prescription program, and to run the program, complete all of the following steps:
[0052] 18 f - Separation: Same as above.
[0053] 18 f - Drying / Activation: Same as above.
[0054] 18 F labeling reaction: transfer 1ml of the precursor 3-O-(methoxymethyl)-16,17-O-sulfonyl-16-estradiol in anhydrous acetonitrile solution (2mg / ml) to the reaction bottle, 100°C Heating, closed reaction 12min.
[0055] Hydrolysis reaction: Transfer 1.0ml of 0.2M HCl in acetonitrile solution to the reaction bottle, heat at 90°C, seal the reaction for 8min; then transfer and add 1.5ml 0.2M NaHCO 3 Solution neutralization.
[0056] Separation and purification: transfer the neutralized solution through Al 2 o 3 Column, injected into the injection loop of semi-preparative HPLC, run HPLC, the separation column is C18 colum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com