Method for preparing propylene from methanol
A technology for the production of propylene and propylene from methanol, which is applied in chemical instruments and methods, purification/separation of hydrocarbons, and production of hydrocarbons from oxygen-containing organic compounds. It can solve the problems of short catalyst regeneration cycle, difficult catalyst control, and low propylene yield, etc. problems, achieve the effect of shortening the operation cycle, increasing the yield of propylene, and improving the selectivity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
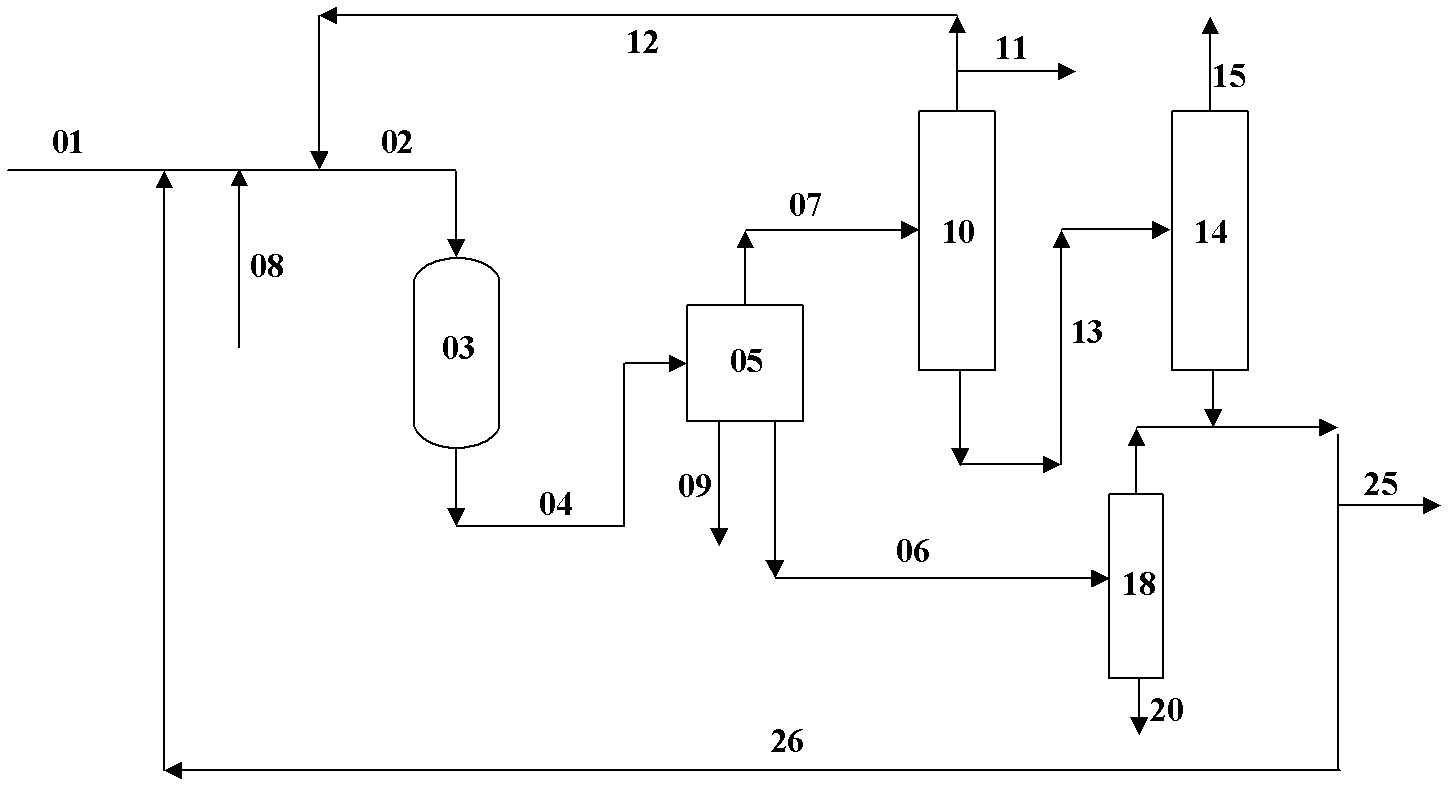
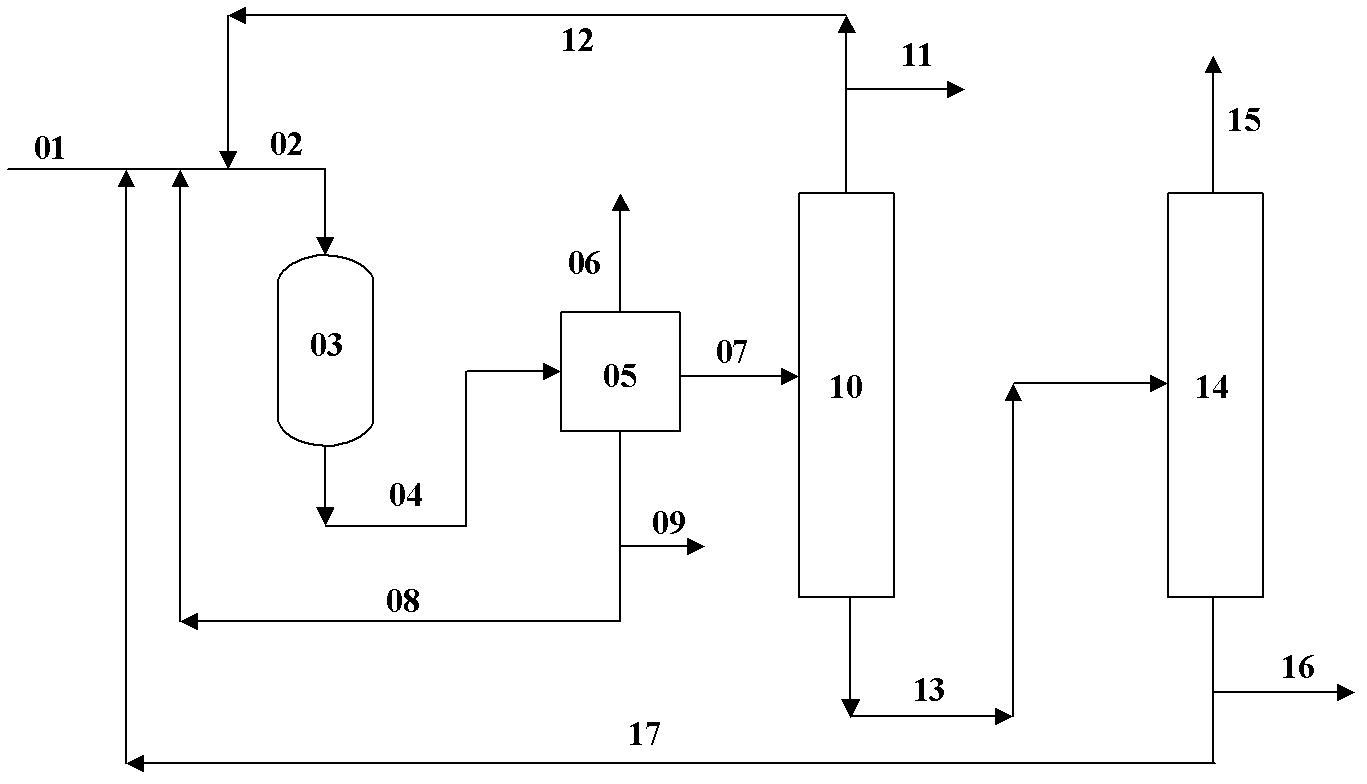
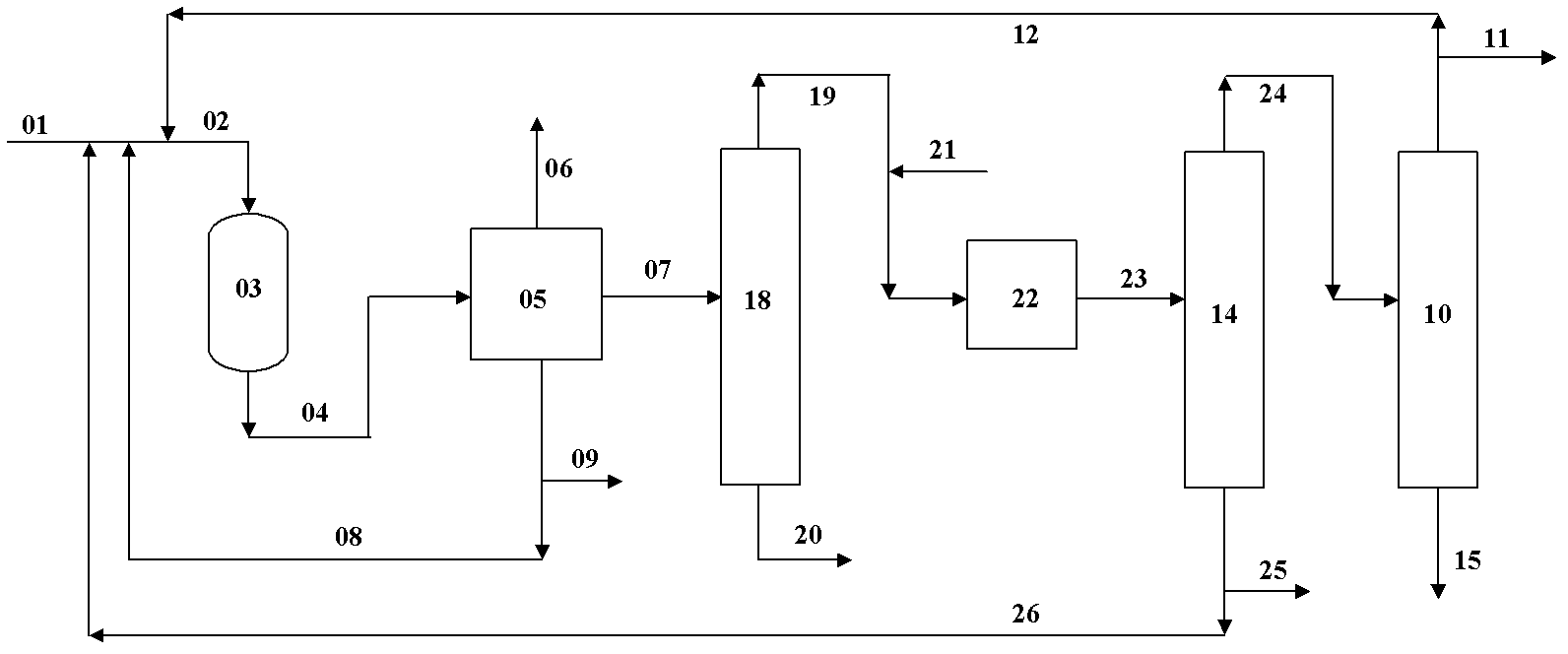
Image
Examples
Embodiment 1
[0037] according to image 3 As shown, the methanol to propylene reaction uses ZSM-5 catalyst, the reaction pressure is 0.1MPa(G), the reaction inlet temperature is 450°C, and the reaction weight space velocity is 6 hours -1 , C 2 and the following logistics and C 4 ~C 5 The circulation ratio of the stream is 0.9; the hydrogenation reaction uses a nickel-based catalyst, the reaction pressure is 2.0MPa(G), the reaction inlet temperature is 50°C, and the reaction weight space velocity is 1 hour -1 , add hydrogen to ensure that the excess hydrogen is 25% (molar ratio). Feed stream 01 composition (weight %) is: 20% methanol, 57.5% dimethyl ether, 22.5% water; 4 ~C5 The composition of stream 26 (% by weight): 74.6% C 4 (excluding alkynes and dienes), 25.4% C 5 (excluding alkynes and dienes), 50 ppm alkynes and dienes; cyclic C 2 The composition (% by weight) of the following stream 12 is: 66.8% C 2 , 32.9% methane, 0.2% hydrogen, 0.1% CO X . After adopting the method of t...
Embodiment 2
[0039] according to image 3 As shown, the methanol to propylene reaction uses ZSM-5 catalyst, the reaction pressure is 0.07MPa(G), the reaction inlet temperature is 430°C, and the reaction weight space velocity is 2 hours -1 , C 2 and the following logistics and C 4 ~C 5 The circulation ratio of the stream is 1.5; the hydrogenation reaction uses a nickel-based catalyst, the reaction pressure is 1.5MPa(G), the reaction inlet temperature is 70°C, and the reaction weight space velocity is 1 hour -1 , add hydrogen to ensure that the excess hydrogen is 20% (molar ratio). Feed stream 01 composition (weight) is: 15% methyl alcohol, 47.5% dimethyl ether, 37.5% water; 4 ~C 5 The composition (weight) of stream 26 is: 74.6% C 4 (excluding alkynes and dienes), 25.4% C 5 (excluding alkynes and dienes), 50 ppm alkynes and dienes; cyclic C 2 The composition (weight) of following stream 12 is: 53.5%C 2 , 43.5% methane, 0.7% hydrogen, 0.3% CO X . After adopting the method of the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com