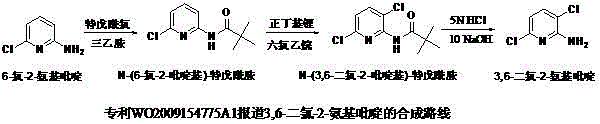
Chemical synthesis method of 3, 6-dichloro-2-aminopyridine
The technology of an aminopyridine and a synthesis method, which is applied in the synthesis field of halogenated aminopyridines, can solve the problems of harsh anhydrous and oxygen-free process conditions, harsh process operation conditions, and difficulty in large-scale production, and achieves good economic and social benefits. , the effect of good reaction selectivity and low manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 45.6 grams (0.25 mol) of 2,3,6-trichloropyridine into a 1000ml pressure-resistant reactor, add 340 grams of 25% ammonia water (containing 5 mol of ammonia), and then add 4.56 grams of catalyst (catalyst composition: 3.56 grams of chlorine cuprous chloride, 0.5 g of benzyltriethylammonium chloride, and 0.5 g of sodium iodide) and seal the reactor, heat it to 120-125°C, react for 5 hours, release unreacted ammonia while hot, and wait for the reaction liquid to After the ammonia gas is released, cool down to room temperature, add 300ml of chloroform, stir, and filter. The obtained filtrate is phase-separated, the organic phase is washed with water, dried with anhydrous magnesium sulfate, and the chloroform is removed under reduced pressure to obtain brown 3,6-dichloro-2-amino Crude pyridine. The crude product was recrystallized with 260ml of toluene, and dried to obtain 37.3 grams of off-white 3,6-dichloro-2-aminopyridine, with a content of 97.8% and a yield of 91.6%. ...
Embodiment 2
[0028] Catalyst is 3.56 gram cuprous chlorides, other is with embodiment 1.
[0029] According to the operation in Example 1, 32.9 g of off-white 3,6-dichloro-2-aminopyridine was obtained, with a content of 96.9% and a yield of 80.9%.
Embodiment 3
[0031] The catalyst is 4.06 grams (the catalyst is composed of: 3.56 grams of cuprous chloride, 0.5 grams of benzyltriethylammonium chloride).
[0032] According to the operation in Example 1, 36.3 grams of off-white 3,6-dichloro-2-aminopyridine was obtained, with a content of 97.2% and a yield of 89.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com