Calcium phosphate nano-structures and preparation method thereof
A nanostructure, calcium phosphate technology, applied in the field of preparation of calcium phosphate nanomaterials, can solve problems such as environmental pollution and increase production costs, and achieve the effects of environmental friendliness, convenient operation and good biodegradation performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
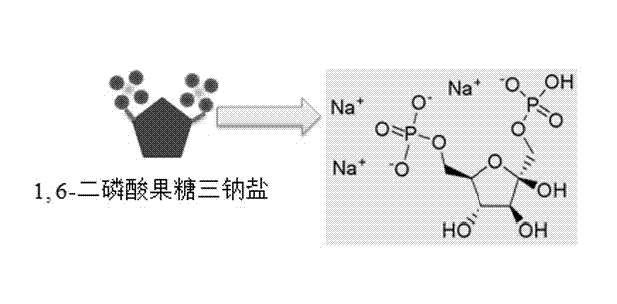
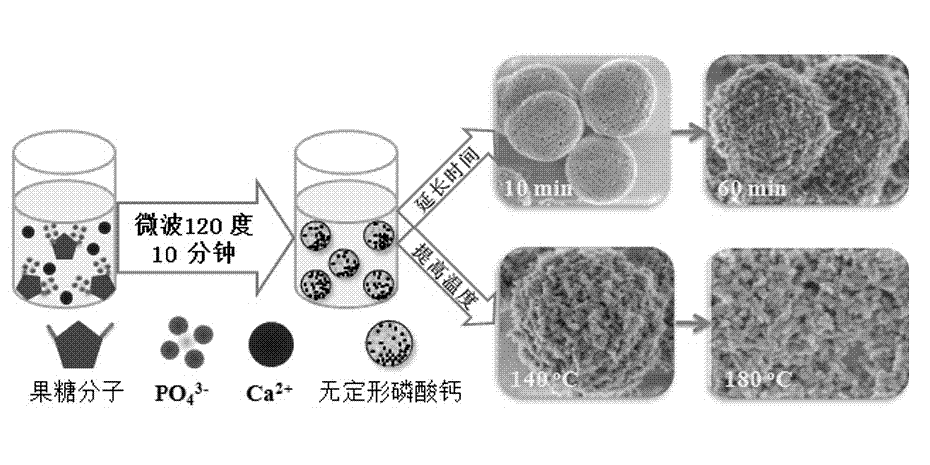
[0049] At room temperature, 0.111 g of CaCl 2 Dissolve in 30 ml of deionized water to form liquid A, and dissolve 0.122 g of trisodium fructose diphosphate in 10 ml of deionized water to form liquid B. After adjusting liquid A with 1 mol / L sodium hydroxide to make its pH equal to 10, add liquid B dropwise, and use magnetic stirring during this process to keep the pH at around 10. After the dropwise addition was completed, the mixed clear solution was transferred into a microwave reactor (capacity 60 ml) and reacted at 100 °C for 10 min. After the reaction system was naturally cooled to room temperature, the product was taken out and centrifuged. The separated product was washed three times with deionized water, once with absolute ethanol, and dried in air at 60°C to obtain Figure 4 For the shown calcium phosphate nanosphere powder, the average diameter of the calcium phosphate nanosphere is 300 nanometers.
Embodiment 2
[0051] At room temperature, 0.111 g of CaCl 2 Dissolve in 30 ml of deionized water to form liquid A, and dissolve 0.122 g of trisodium fructose diphosphate in 10 ml of deionized water to form liquid B. After adjusting liquid A with 1 mol / L sodium hydroxide to make its pH equal to 10, add liquid B dropwise, and use magnetic stirring during this process to keep the pH at around 10. After the dropwise addition was completed, the mixed clear solution was transferred to a microwave reactor (capacity 60 ml) and reacted at 120 °C for 10 min. After the reaction system was naturally cooled to room temperature, the product was taken out and centrifuged. The separated product was washed three times with deionized water, once with absolute ethanol, and dried in air at 60°C to obtain Figure 5 For the calcium phosphate nanosphere powder shown, the surface of the calcium phosphate nanosphere is smooth and contains a porous structure, and the diameter of the pores is below 40 nanometers. s...
Embodiment 3
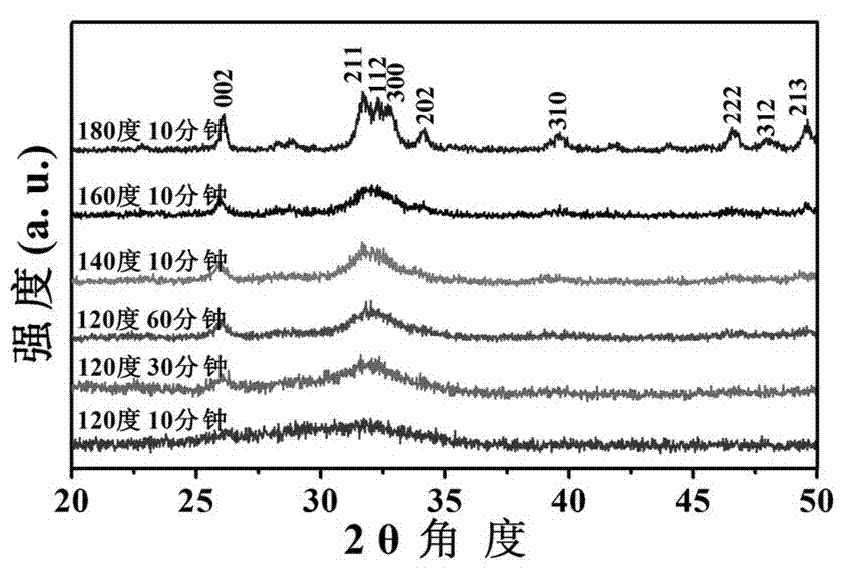
[0054] At room temperature, 0.111 g of CaCl 2 Dissolve in 30 ml of deionized water to form liquid A, and dissolve 0.122 g of trisodium fructose diphosphate in 10 ml of deionized water to form liquid B. After adjusting liquid A with 1 mol / L sodium hydroxide to make its pH equal to 10, add liquid B dropwise, and use magnetic stirring during this process to keep the pH at around 10. After the dropwise addition was completed, the mixed clear solution was transferred into a microwave reactor (capacity 60 ml) and reacted at 120 °C for 30 min. After the reaction system was naturally cooled to room temperature, the product was taken out and centrifuged. The separated product was washed three times with deionized water, once with absolute ethanol, and dried in air at 60°C to obtain Figure 8 For the calcium phosphate nanosphere powder shown, the average diameter of the calcium phosphate nanosphere is 500 nanometers, and the surface is relatively rough. see figure 2 It can be seen t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com