UGT1A9/1A8 specific probe substrate and use thereof
A technology for probe substrates and compounds, applied in the field of medicine, can solve problems such as lack of specific probe substrates, and achieve the effects of easy availability, high safety, and high LD50 value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
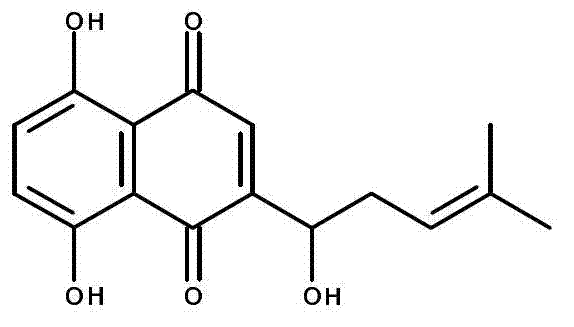
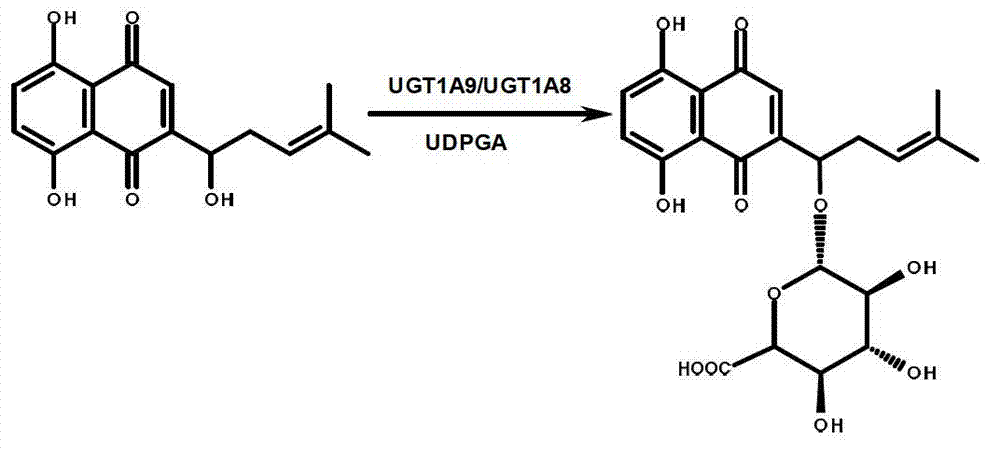
[0028] Example 1: Shikonin is used for the quantitative determination of the enzyme activity of UGT1A9 in the recombinant single enzyme
[0029] (1) Prepare 180 μl UGT metabolic reaction system in advance, including Tris-HCl buffer (5mM) at pH 7.4, 5mM MgCl 2 , recombinant human UGT1A9 (0.015mg / ml), the final concentration of shikonin is 35μM, pre-incubated at 37°C for 5 minutes;
[0030] (2) Add 20 μl of 40 mM UDPGA (final concentration: 4 mM) to the reaction system to initiate the reaction; (3) After 20 minutes, add 100 μl of ice-cold methanol and shake vigorously to terminate the reaction;
[0031] (4) Use a high-speed refrigerated centrifuge at 4 °C, 20,000×g, after high-speed centrifugation for 20 minutes, take the supernatant for UFLC-UV detection;
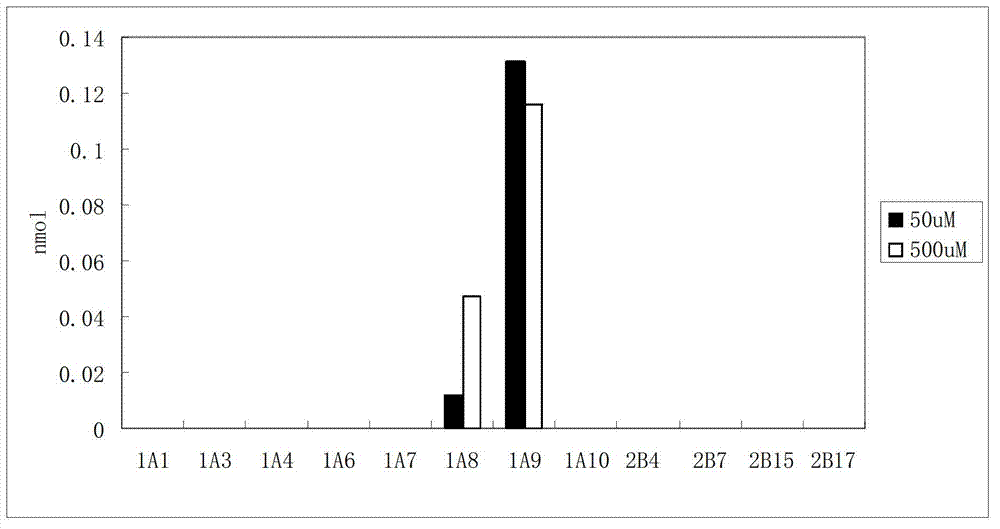
[0032](5) Quantitative detection of shikonin and its glucuronidation products at 517nm. The calculated maximum catalytic rate of recombinant human UGT1A9 enzyme was 150.3 pmol / mim / mg.
Embodiment 2
[0033] Example 2: Shikonin is used to quantitatively determine the enzyme activity of UGT1A9 in human liver microsomes
[0034] (1) The reaction system is the same as above. The UGT1A9 in the system was replaced with human liver microsomes, the protein concentration of the microsomes was 0.025 mg / ml, and the reaction time was 20 minutes.
[0035] (2) After high-speed centrifugation at 4°C and 20,000×g for 20 minutes, take the supernatant for UFLC-UV detection;
[0036] (3) Quantitative detection of shikonin and its glucuronidation products at 517nm. The maximum catalytic rate of UGT1A9 in human liver microsomes was measured to be 92.6 pmol / min / mg.
Embodiment 3
[0037] Embodiment 3: detect the enzymatic activity in recombinant human UGT1A8 single enzyme
[0038] The reaction conditions and operation process are the same as those in Example 1, except that UGT1A8 is used instead of UGT1A9 for shikonin metabolism incubation. The same method can be used to measure the activity of different batches of recombinant human UGT1A8. The maximum reaction rate of recombinant human UGT1A8 determined in this experiment was 9.67pmol / min / mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com