A kind of oral solid pharmaceutical composition containing dronedarone hydrochloride and preparation method thereof
A technology of dronedarone hydrochloride and a composition, which is applied in the field of oral solid pharmaceutical compositions, can solve problems such as the stability of the solid pharmaceutical composition needs to be investigated, and achieves the effects of improving bioavailability, simple and feasible preparation process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
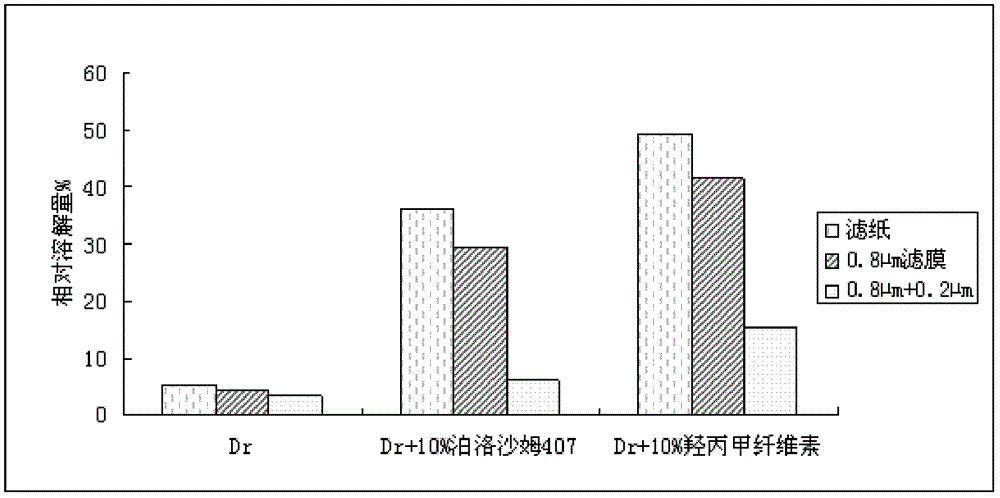
[0032] According to the test method of "the maintenance test of the active ingredient itself in the pH6.7 solution" provided by the patent CN1091593C, an experiment was designed to investigate the solubility of poloxamer 407 and hydroxypropyl methylcellulose to dronedarone hydrochloride. influences.
[0033] A 2 mg / ml solution of dronedarone hydrochloride was prepared in a pH 4.5 phosphate buffer solution at 37° C. for 2 hours, and filtered through a 0.8 μm filter membrane. The active ingredients in the solution were measured by UV spectroscopy, and the dissolved amount of the main drug in each sample was calculated. The specific results are shown in Table 2.
[0034] The dissolved amount of dronedarone hydrochloride in pH4.5 phosphate buffer solution of table 2
[0035] sample
Dissolution percentage %
74.6
Dronedarone Hydrochloride + 10% Poloxamer 407
73.9
Dronedarone Hydrochloride + 10% Hydroxypropyl Methyl...
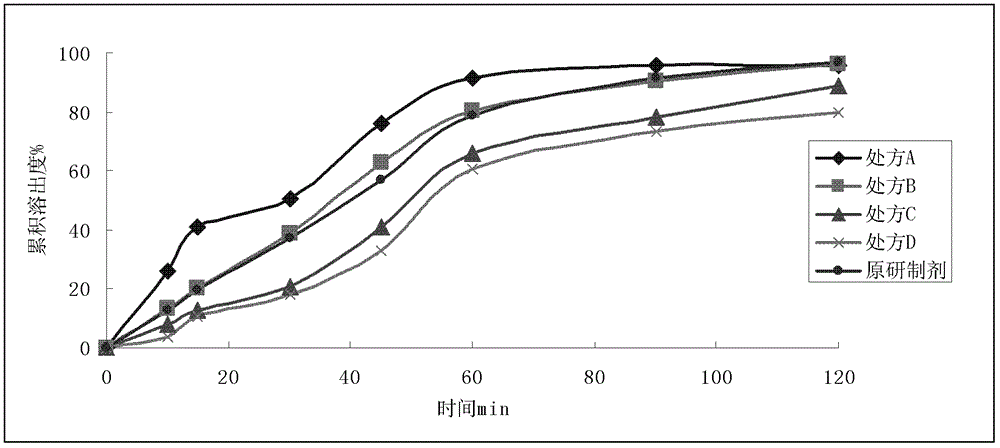
Embodiment 2
[0043]
[0044] Preparation Process:
[0045] Prescriptions A to D all adopt the wet granulation process; mix micronized dronedarone hydrochloride, hydroxypropyl methylcellulose, lactose, and starch evenly, use 50% ethanol to make soft materials, and make wet granules through a 18-mesh sieve. After drying, 20-mesh sieve is used for sizing, and crospovidone, silicon dioxide and magnesium stearate are added externally, mixed evenly, and compressed into tablets.
[0046] With a concentration of 15% Opadry 85GII68918 aqueous solution as the coating solution, the sample cores of the prescriptions A to D in Example 2 were coated to obtain a self-made sample, which was dissolved together with the original preparation in Appendix XC of Part Two of the Chinese Pharmacopoeia 2010 Edition In the second method of the degree measurement method, the dissolution medium is 1000mLpH4.5PBS, the rotation speed is 75r / min, and UV is measured under the conditions of 10, 15, 30, 45, 60, 90, and ...
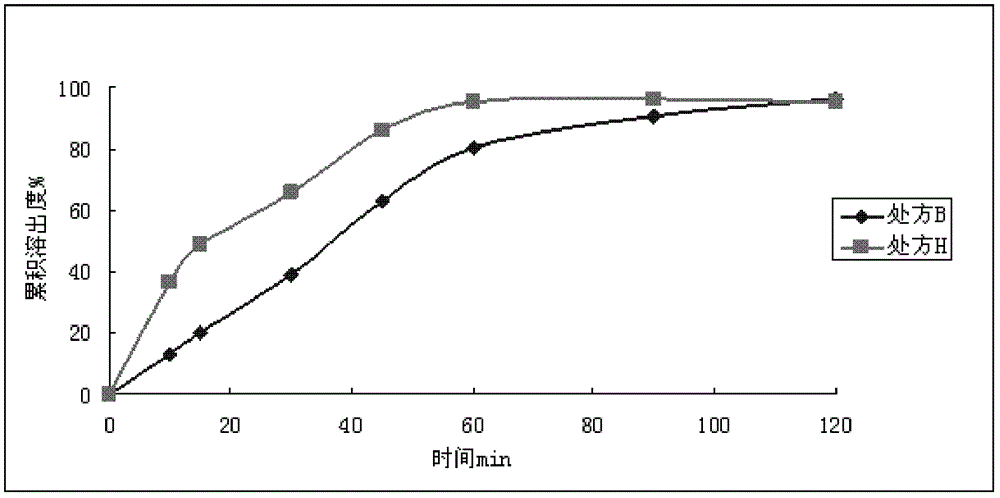
Embodiment 3
[0049]
[0050] Preparation Process:
[0051] Prescriptions E and F: mix micronized dronedarone hydrochloride, hydroxypropyl methylcellulose, lactose, and starch evenly, use 30% ethanol to make soft materials, and make wet granules through a 18-mesh sieve. After drying, 20-mesh sieve is sized, and crospovidone, silicon dioxide and magnesium stearate are added externally, and mixed evenly. Prescription E prepares granules and packs them separately. The granules prepared by formulation F were filled into capsules.
[0052] The prescription E granule adopts the second method in the dissolution test method of the second appendix XC of the "Chinese Pharmacopoeia" 2010 edition, and the prescription F capsule adopts the first method in the dissolution test method of the second appendix XC of the "Chinese Pharmacopoeia" 2010 edition. The dissolution medium is 1000mLpH4.5PBS, rotating speed 75r / min, UV measurement under the conditions of 10, 15, 30, 45, 60, 90, 120min respectively...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com