Fullerene derivative containing double-benzene nucleus and preparation method and application thereof
A technology for naphthalene ring fullerenes and derivatives is applied in the fields of fullerene derivatives and their preparation and application, which can solve the problems of high synthesis temperature and limited application, and achieves simple synthesis process, high open circuit voltage, and high electron mobility. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, preparation contains naphthalene ring fullerene derivative F1
[0028] The synthetic route is as follows:
[0029]
[0030] Compound 1,2-bis(bromomethyl)naphthalene and 0.1 mmol fullerene C shown in 0.25 mmol formula II 60 Dissolve in toluene, add 0.1mmol potassium iodide and 0.05mmol 18-crown-6 ether to react at 80 ° C for 24 hours, pour the reaction solution into water, extract the aqueous phase with toluene, and dry the organic phase with anhydrous sodium sulfate. The organic solvent was removed by rotary evaporation, followed by silica gel column separation (petroleum ether:toluene=1:1.2, v / v) to obtain the powdery solid product F1 with a yield of 72%.
[0031] 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 7.19-8.58 (m, 12H), 4.12-4.78 (m, 8H).
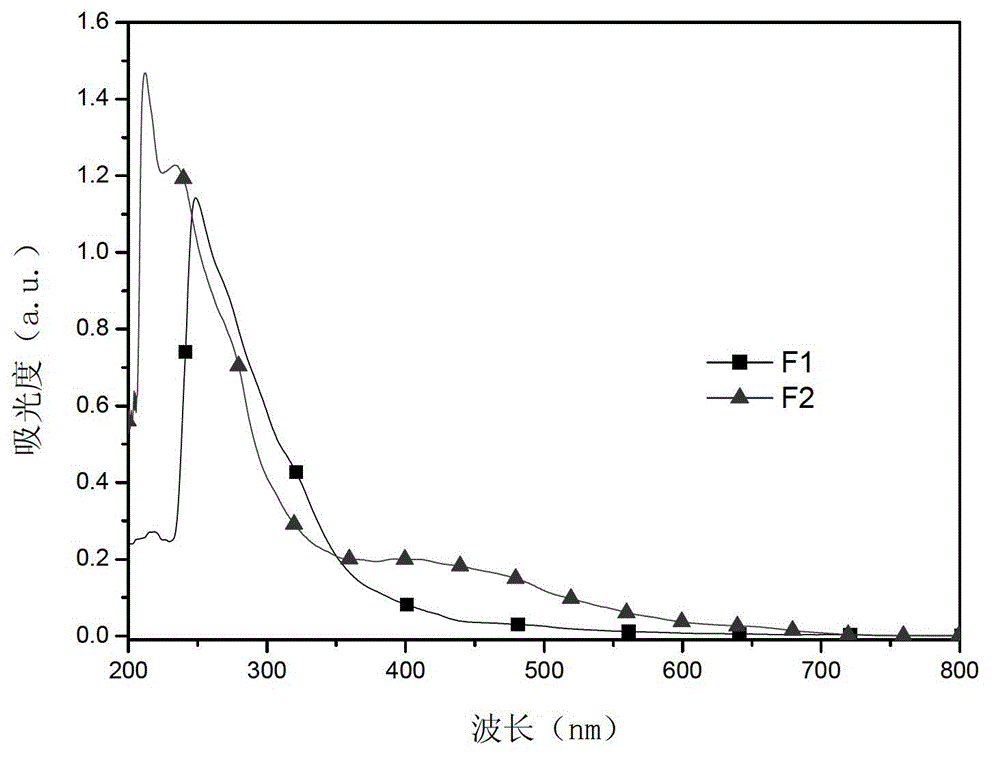
[0032] The mass spectrum characterization of product F1 is shown in Table 1, and the measured value is close to the theoretical molecular weight; the absorption spectrum of product F1 in tetrahydrofuran is shown i...
Embodiment 2
[0035] Embodiment 2, preparation contains naphthalene ring fullerene derivative F2
[0036] The synthetic route is as follows:
[0037]
[0038] 0.1 mmol fullerene C containing 70 carbons 70 Dissolve 0.25mmol 1,2-bis(bromomethyl)naphthalene in toluene, add 0.1mmol potassium iodide, react for 2 hours at 150°C, pour the reaction solution into water, extract the aqueous phase with toluene, and wash the organic phase with anhydrous Dry over sodium sulfate, remove the organic solvent by rotary evaporation, and separate through a silica gel column (petroleum ether:toluene=1:1.5, v / v) to obtain the powdery solid product F2 with a yield of 75%.
[0039] 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 7.18-8.64 (m, 12H), 4.37-5.35 (m, 8H).
[0040] The mass spectrum characterization of product F2 is shown in Table 1, and the measured value is close to the theoretical molecular weight; the absorption spectrum of product F2 in tetrahydrofuran is shown in figure 1 , has good absorption performan...
Embodiment 3
[0041] Characterization of polymer solar cell device performance prepared by fullerene derivatives F1 and F2 prepared in embodiment 3, embodiment 1 and 2
[0042] A 25-nm thick layer of poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) was spin-coated on cleaned indium tin oxide (ITO) conductive glass, where PEDOT : The molar ratio of PSS is 1:1, and then the fullerene derivatives and poly(3-hexylthiophene) (P3HT) prepared in Examples 1 and 2 are dissolved in o-dichloro In benzene, the total concentration of the solution is 34mg / mL, and the solution is spin-coated on the PEDOT:PSS film surface as an active layer, with a thickness of 150nm, and heat treated at 150°C for 10 minutes; metal Ca is vapor-deposited on the surface of the above-mentioned active layer, The thickness is 20nm, the metal Al is vapor-deposited on the surface of the metal Ca, the thickness is 150nm, and the organic solar cell is obtained.
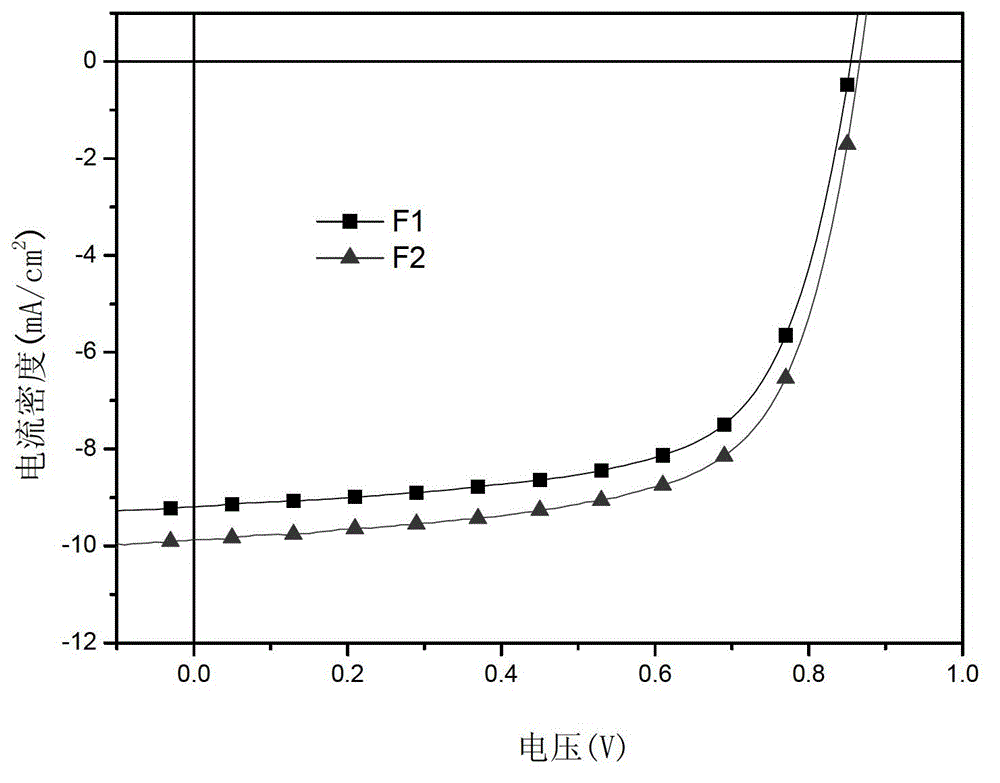
[0043] The performance of the organic solar cell ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com