Method for preparing 2-ethyl-2-hexenoic aldehyde through n-butyl aldehyde self-condensation under catalysis of solid acid
A technology of solid acid catalysis and solid acid catalyst, applied in the field of green chemistry, can solve the problems of large acid consumption, environmental pollution, equipment corrosion, etc., and achieve the effects of high activity, simple selectivity and stability, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] In the 100ml autoclave, put into 40g n-butyraldehyde, then add the Hβ(40) catalyst of 18.0% of n-butyraldehyde quality, use N 2 Air replacement, magnetic stirring, and reaction at 120°C for 4 hours. After the reaction, filter under reduced pressure, and perform gas chromatography analysis on the product liquid. The conversion rate of n-butyraldehyde is 75.5%, and the conversion rate of 2-ethyl-2-hexenal The selectivity was 81.7%.
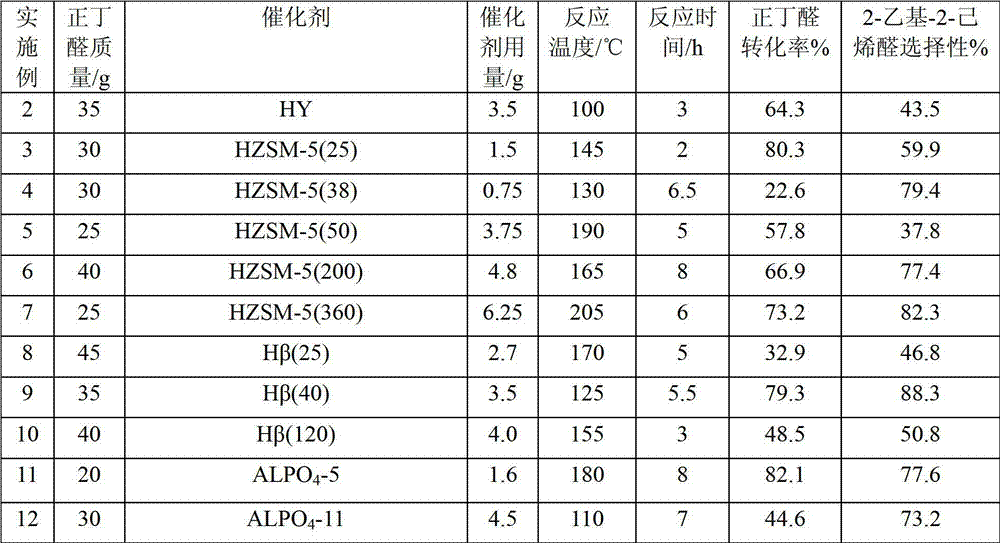
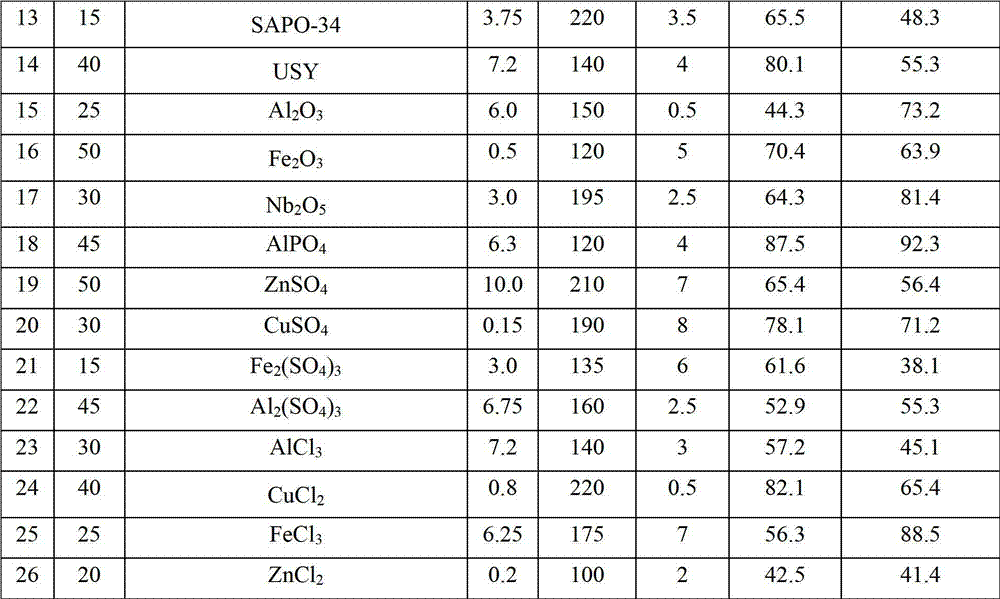
[0015] Examples 2-26 According to the operation steps of Example 1, the changed reaction conditions and results are shown in the summary table.
[0016]
[0017]
Embodiment 27
[0019] In the 100ml autoclave, put 30g n-butyraldehyde, then add the HZSM-5 (150) catalyst of n-butyraldehyde quality 5%, use N 2 Air replacement, magnetic stirring, and reaction at 150°C for 3 hours. After the reaction, filter under reduced pressure, and perform gas chromatography analysis on the filtrate. The conversion rate of n-butyraldehyde is 80.7%. The selection of 2-ethyl-2-hexenal Sex is 88.0%. The reacted HZSM-5(150) catalyst was washed three times with absolute ethanol, dried at 80°C for 4h, put into a muffle furnace, and calcined at 500°C for 4h. Then under the same reaction conditions, the repeated use effect of the catalyst was investigated, and the results are shown in the table below. With the increase of the number of reactions, the activity of the catalyst decreased, and the selectivity of 2-ethyl-2-hexenal only decreased by 6.7% after being used for 4 times, and the catalyst still had a high activity.
[0020] Catalyst reuse times
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com