Process for producing alkyl nitrite
The technology of an alkyl nitrite and process is applied in the field of preparing the alkyl nitrite, can solve the problems of environmental pollution, low yield of the alkyl nitrite and the like, and achieves the effects of improving the reaction rate and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
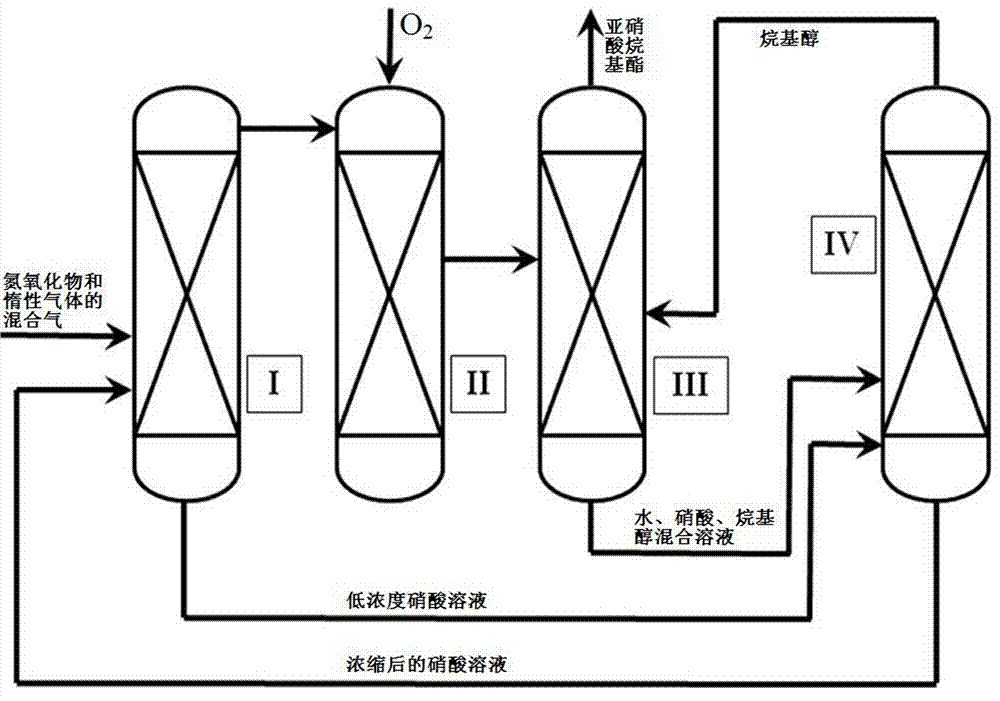
[0047] Contains N 2 and NO mixed gas (the total gas flow rate is 284 mL / min, where N 2 volume fraction is 77.8%, NO volume fraction is 22.2%) enters the reactor I that is equipped with 50% nitric acid solution (weight percentage), the reaction pressure of reactor I is 0.1 MPa, and reaction temperature is 30 o C, the contact time of mixed gas and nitric acid solution in reactor I is 1 second. The tail gas after the reaction enters the reactor II through the pipeline, and the O 2 reaction, and keep the reaction O 2 The volume ratio to NO is 0.5. The nitrogen oxides generated after the reaction in the reactor II enter the reactor III through the pipeline, and the reactor III is equipped with an excessive amount of pure ethanol solution. After the nitrogen oxides react with the ethanol solution, the generated ethyl nitrite is discharged from the pipeline Collection, wherein the generation rate of ethyl nitrite is 103.5 mmol / h.
[0048] The nitric acid solution after the react...
Embodiment 2
[0050] The O introduced into the reactor II 2 The amount is reduced to 0, i.e. the O in the reactor II is maintained in the reaction 2 The volume ratio to NO is 0, and other conditions are the same as those in Embodiment 1. The formation rate of ethyl nitrite was 44.1 mmol / h.
Embodiment 3
[0052] Contains N 2 and NO mixed gas (the total gas flow rate is 284 mL / min, where N 2 The volume fraction is 77.8%, the NO volume fraction is 22.2%) enters the reactor I that is equipped with 15% nitric acid solution (weight percentage), the reaction pressure of reactor I is 0.5 MPa, and reaction temperature is 90 o C, the contact time of mixed gas and nitric acid solution in reactor I is 50 seconds. The tail gas after the reaction enters the reactor II through the pipeline, and the O 2 reaction, and keep the reaction O 2 The volume ratio to NO is 0.2. The nitrogen oxide enters the reactor III through the pipeline, and the excess pure ethanol solution is installed in the reactor III. After the nitrogen oxide reacts with the ethanol solution, the generated ethyl nitrite is discharged and collected through the pipeline, and the generation of ethyl nitrite The rate was 94.5 mmol / h.
[0053] The nitric acid solution after the reaction in the reactor I and the solution after ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com