Protease with improved thermal stability as well as construction method and application thereof
A thermal stability, keratinase technology, applied in the field of genetic engineering, can solve the problems of polluting the environment, reducing the nutritional value of products, destroying amino acids, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The keratinase that embodiment 1 thermostability improves
[0018] The keratinase of the present invention is based on the gene sequence published by GenBank accessionnos.JX504681, and amino acid mutations occur at four sites in its mature region, specifically N122Y, N160C, A193P, and N217S, which can be chemically synthesized or site-directed mutation Amino acid substitutions were carried out at four sites in the mature region.
Embodiment 2
[0019] Construction and identification of embodiment 2 producing keratinase genetically engineered bacteria
[0020] The ker gene encoding keratinase in Example 1 was obtained by chemical total synthesis method, cloned into pMA0911, and the recombinant expression plasmid pMA 0911-kerTB containing the kerTB gene was obtained.
[0021] The recombinant plasmid pMA 0911-kerTB transformed Bacillus subtilis WB600 competent cells to obtain a genetically engineered bacterium that can grow normally on LB plates containing kanamycin (30 mg / mL), and was identified and named Bacillus subtilis WB600-pMA 0911- kerTB. .
Embodiment 3
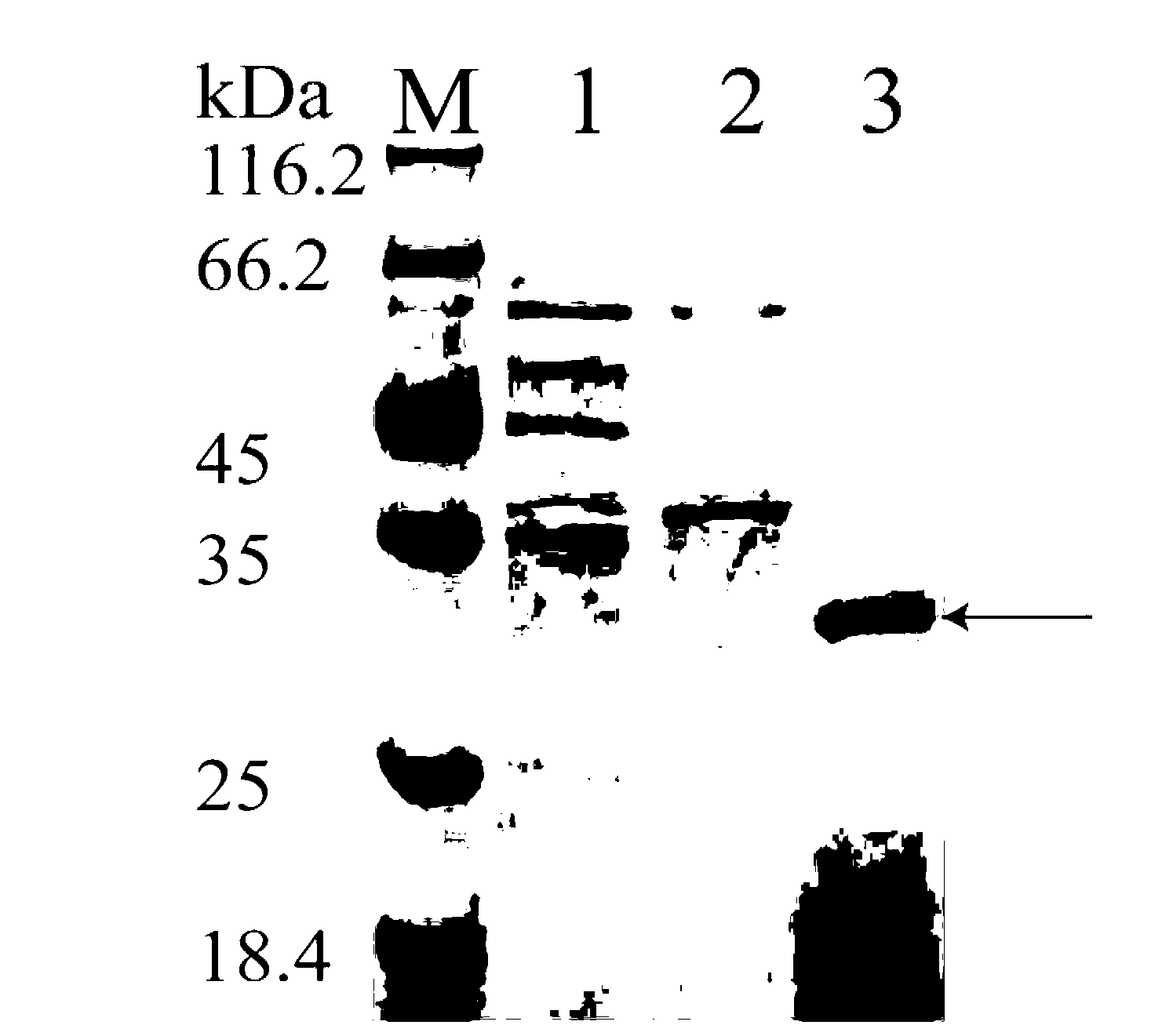
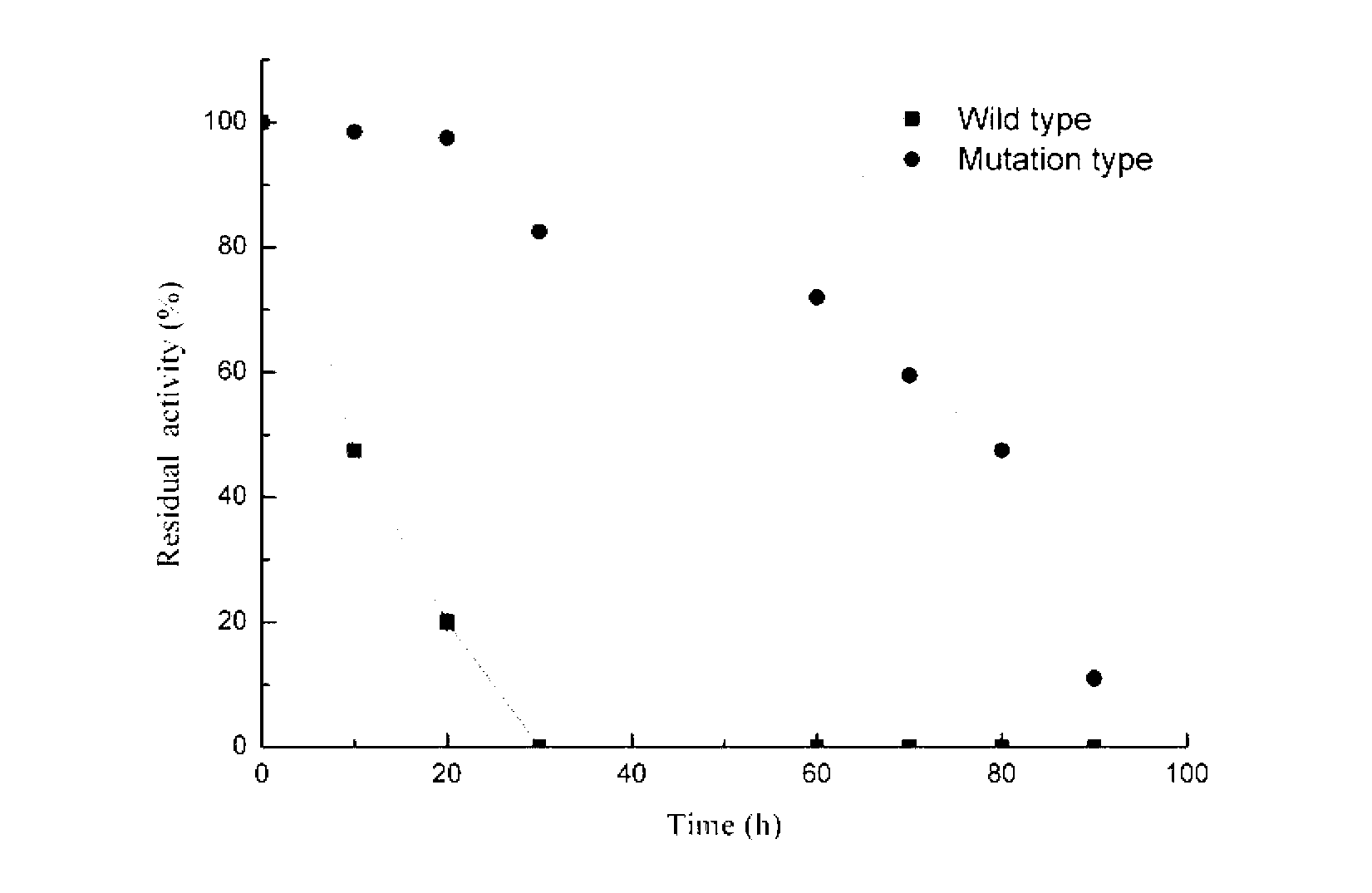
[0022] Enzyme activity assay and protein electrophoresis of embodiment 3 recombinant bacteria
[0023] Keratinase enzyme activity assay method: Centrifuge the fermentation broth for 10min (10000×g, 4°C) to take the fermentation supernatant, absorb 200uL of appropriately diluted enzyme solution, add 300uL 0.05mol / L gly-NaOH buffer solution (pH9.0) Dissolve 1% of the substrate (keratin), incubate at 50°C for 20min, add 500uL 4M TCA solution to terminate the reaction. Centrifuge for 10min, draw 200uL supernatant and transfer it to a new test tube, then add 1mL Folinol reagent and 200uL 0.5M Na 2 CO 3 Then develop color at 50 degrees for 15 minutes. The blank is to add 500uLTCA solution at the same time as adding the enzyme solution, and the filtered supernatant after the same process reaction is used as a blank. The absorbance was detected at 660nm, and according to the definition of the tyrosine standard curve, the release of 1ug of tyrosine in every 15min was defined as an e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com