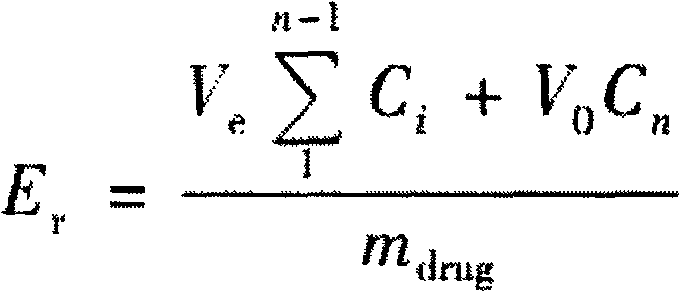Transdermal absorption pharmaceutical composition containing testosterone
A composition and drug technology, applied in the field of pharmaceutical composition, can solve the problem of uncountable combinations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0018] The particle size in the following examples refers to the corresponding particle size when the cumulative particle size distribution percentage reaches 90%, which are all measured according to the light scattering method in Appendix 73 of the Chinese Pharmacopoeia 2010 Edition.
[0019] In vitro percutaneous penetration test
[0020] After 3-month-old healthy rats were anesthetized and killed, the abdominal hair was removed with scissors, the uninjured skin was removed, and the subcutaneous tissue was removed. After cleaning, they were respectively fixed at the release port of the Franz diffusion cell, and pH 7.4 was added to the receiving chamber. A mixture of phosphate-buffered saline (PBS), propylene glycol (1:1) and gentamicin sulfate (50 μg / ml) was used as the release medium, and the endothelial layer was kept in close contact with the solution. 10 units of the medicine taken each time were subjected to the permeability test.
[0021] Take 0.1ml of the drug soluti...
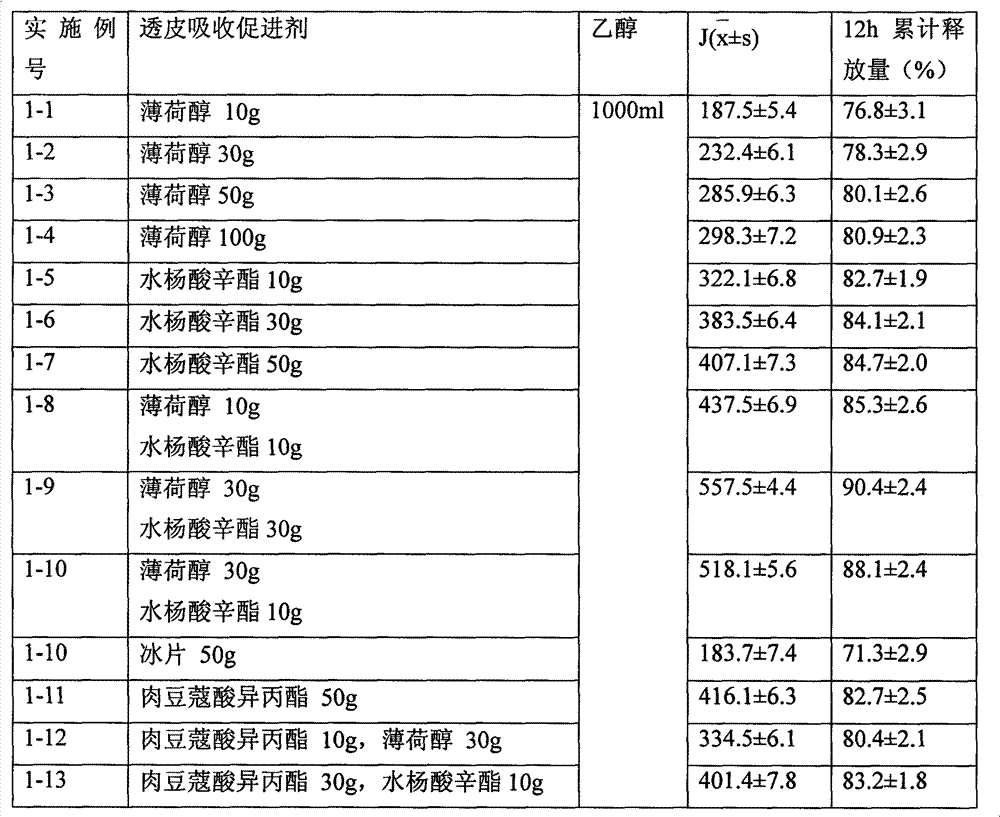
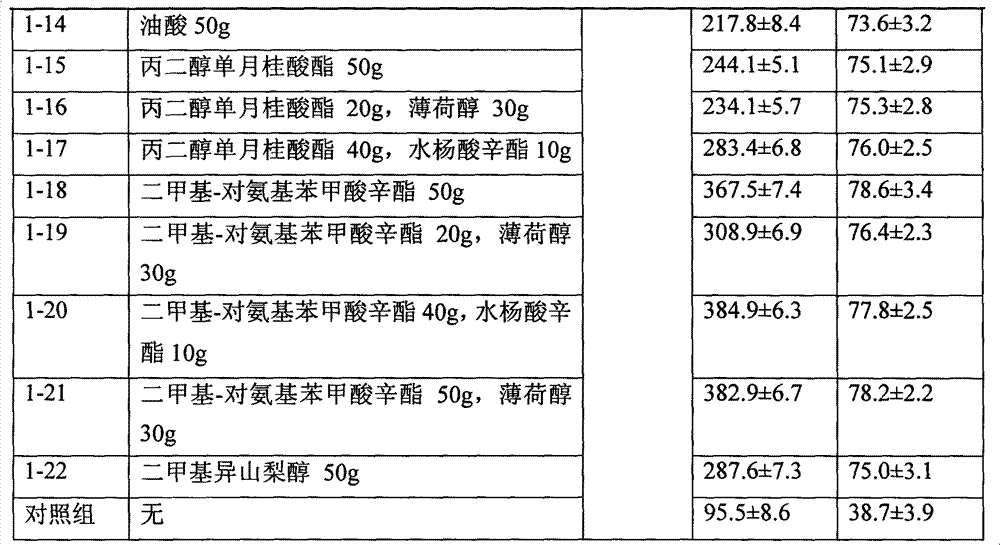
Embodiment 1
[0026]
[0027]
[0028] Dissolve the prescribed amount of transdermal absorption enhancer in 400ml of ethanol, dissolve 20g of testosterone (average particle size of 5μm) in 500ml of ethanol, mix the two liquids evenly, filter with No. 3 vertical melting funnel, and wash No. 3 with the remaining ethanol. Vertical melting funnel, above-mentioned preparation is filled in the medicinal spray bottle according to the requirement of testosterone 0.1g / bottle.
[0029] The permeability test was carried out according to the requirements of the above-mentioned in vitro percutaneous penetration test, and the penetration rate of 3h and the cumulative release amount of 12h were obtained. The experimental results proved that the effect of the penetration enhancer mixed with menthol and octyl salicylate was better than that used alone, far better other penetration enhancers.
Embodiment 2
[0031] The average particle size of the drug in this example is 5 μm, and the dosage is equivalent to 20 g of testosterone.
[0032] 90-day stability test:
[0033] The pharmaceutical composition obtained in the examples is stored airtight at 60°C ± 2°C, and the relative humidity is 75% ± 5%, and the stability test is carried out, and the drug content of 5 units is respectively detected on the 0th day and stored until the 90th day , A=average content on day 90 / average content on day 0.
[0034]
[0035]
[0036] Take the prescribed amount of transdermal absorption enhancer and dissolve it in 400ml of liquid alcohol, dissolve the drug in 500ml of liquid alcohol, mix the two liquids evenly, filter with No. 3 vertical melting funnel, wash No. 3 vertical melting funnel with the remaining liquid alcohol, and put The above-mentioned preparation is filled in a medicinal spray bottle according to the requirement of 0.1 g / bottle (equivalent to testosterone) of the drug, and then...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com