Application of SO3H- functionalized ionic liquid serving as catalyst to synthesis of 2-acetylthiophene
A SO3H-, 1.SO3H- technology, applied in the direction of organic chemistry, can solve the problems of high catalyst toxicity, large amount of three wastes, high cost, and achieve the effects of easy industrial production, high catalyst activity, and designable structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
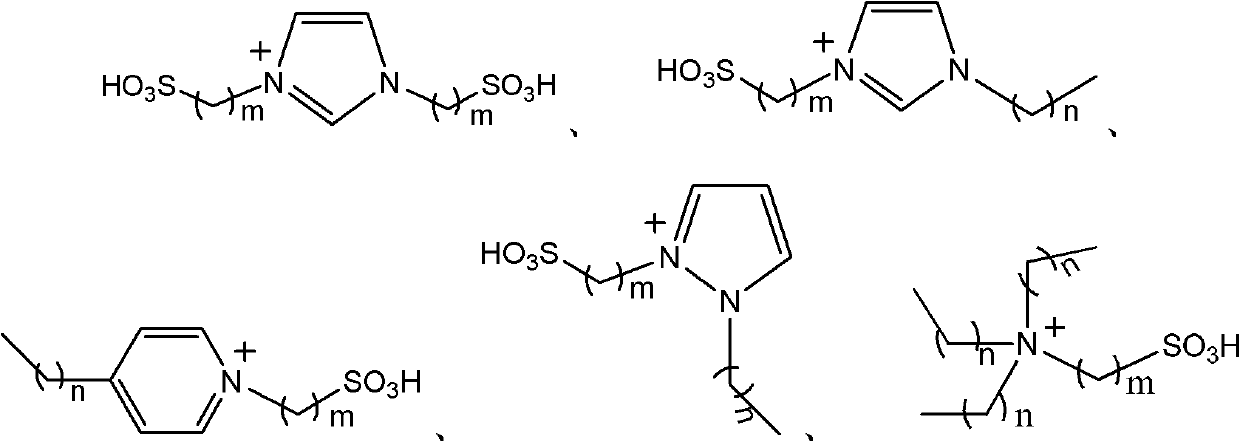
[0023] The ionic liquid [(HSO 3 -p) 2 im)][NTf 2 ] (0.165mmol), thiophene (10.4mmol), acetic anhydride (the amount in Table 1) was added to a 100ml round-bottomed flask equipped with a thermometer and reflux condenser, stirred magnetically, and reacted for 2h under normal pressure at 30℃ , And then stand and cool to room temperature, the reaction solution was distilled under reduced pressure to separate unreacted thiophene and acetic anhydride.
[0024] Table 1. The effect of reactant ratio on the reaction performance of thiophene and acetic anhydride
[0025]
[0026] It can be seen from the data in Table 1, the ionic liquid [(HSO 3 -p) 2 im)][NTf 2 ] Is a catalyst. When the amount of catalyst is only 1.6% (mol%) and the reaction temperature is only 30°C, the yield of 2-acetylthiophene can reach 97%, and the selectivity of 2-acetylthiophene is about 99%. The process does not need to add organic solvents and additives.
Embodiment 6-11
[0028] Add different ionic liquids (0.165mmol) as shown in Table 2, thiophene (10.4mmol), acetic anhydride (10.4mmol) into a 100ml round bottom flask equipped with a thermometer and reflux condenser, magnetically stirred, and at 60℃ The reaction was carried out under normal pressure for 2 hours, and then left to stand and cooled to room temperature. The reaction solution was distilled under reduced pressure to separate unreacted thiophene and acetic anhydride.
[0029] Table 2. Comparison of catalytic performance of different ionic liquids:
[0030] Example
[0031] From the data in Table 2, it can be seen that the anion is NTf 2 - SO 3 H-functionalized ionic liquid ([(HSO 3 -p) 2 im][NTf 2 ] And [(HSO 3 -p)im][NTf 2 ]) As a catalyst, when the amount of catalyst is only 1.6% (mol%), the reaction temperature is only 60°C, and thiophene is reacted with acetic anhydride in equal proportions, the yield of 2-acetylthiophene reaches 92%, and 2-acetylthiophene The selectivity reaches a...
Embodiment 12-16
[0033] Add ionic liquid, thiophene (52.4mmol), acetic anhydride (104.8mmol) into a 100ml round-bottomed flask equipped with a thermometer and reflux condenser, stir magnetically, and react at 60℃ for 2h under normal pressure, then stand still After cooling to room temperature, the reaction solution was distilled under reduced pressure to separate unreacted thiophene and acetic anhydride.
[0034] Table 3. The influence of ionic liquid cation structure on the acylation performance of thiophene and acetic anhydride
[0035] Example
[0036] It can be seen from the data in Table 3 that the anion is NTf 2 - SO 3 H-functionalized ionic liquid is used as a catalyst, when the amount of catalyst is only 1.6% (mol%) and the reaction temperature is only 60℃, the yield and selectivity of 2-acetylthiophene are very different with the change of cation structure. The ionic liquid of alkyl sulfonate imidazole cation can make the yield of 2-acetylthiophene reach 99%, and the selectivity of 2-ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com