Five-membered heterocycle modified 4'-spiro nucleoside compounds and application in virus resistance
A nucleoside compound, five-membered heterocycle technology, applied in the field of five-membered heterocycle modified 4'-spirocyclic nucleoside compounds, can solve the problem of lack of specific treatment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Synthesis
[0048] Hydroxylamine (1.53g, 22mmol) was dissolved in 30mL of water and added to a 250mL three-necked flask. Benzaldehyde (2.12 g, 20 mmol) was dissolved in 50 mL of ethanol and added to the above system. 22 mL of 1N NaOH solution was added dropwise to the system with stirring at room temperature. The dropwise addition was completed within half an hour. After the dropwise addition was completed, TLC detected that the raw material disappeared. Ethanol and water were evaporated by rotary evaporation to obtain a light yellow solid, and 20 mL of water was added to dissolve the white solid, and the aqueous solution was extracted with 10 mL of ethyl acetate for three times, and the combined organic phase was dried with anhydrous sodium sulfate and the solvent was evaporated to obtain a crude product 2.18 g of benzaldoxime light yellow solid. Weigh the crude product benzaldoxime (1.21g, 10mmol) obtained in the previous step, dissolve it in 30mL of D...
Embodiment 2
[0052] Example 2 Synthesis
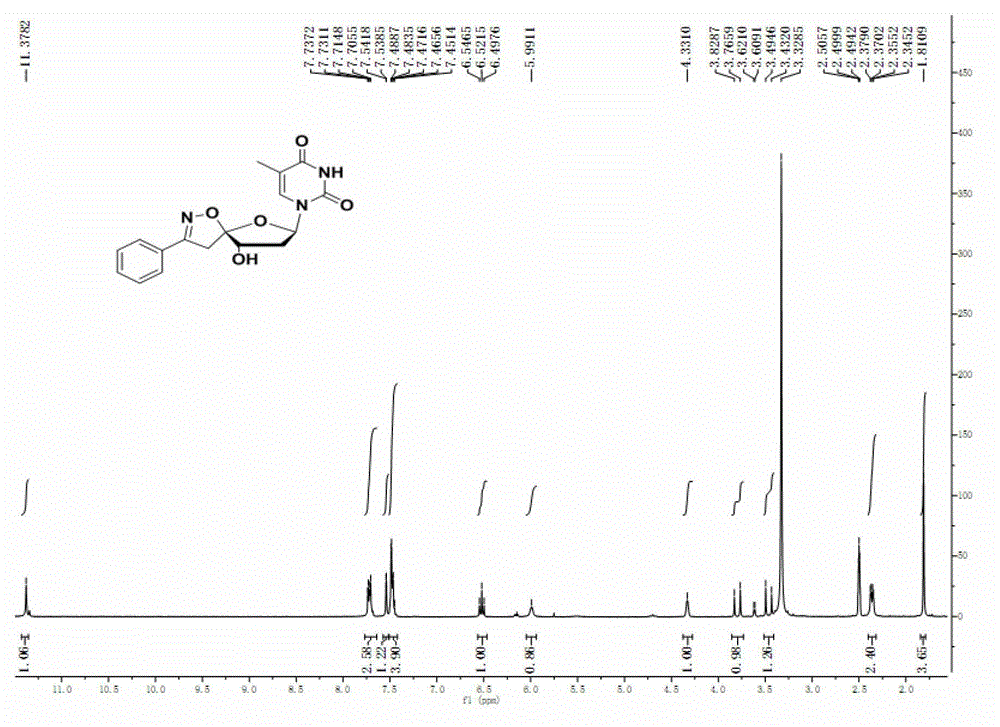
[0053] Take 1.6mmol of the crude product of chlorobenzaldoxime and dissolve it in 20mL of dichloromethane, and add it dropwise to the compound 2′,3′-tert-butyldimethylsilyl-protected-4′,5′-double bond under ice bath Uridine (1.0 mmol) in dichloromethane (5 ml) solution. After the dropwise addition was completed, a solution of triethylamine (0.24 mL, 1.6 mmol) in 10 mL of dichloromethane was added dropwise under ice-cooling. After the reaction was completed, the solvent was evaporated on a water pump and separated by column chromatography. The cyclization product was obtained. 1 H NMR (300MHz, CDCl 3 )δ7.83 (d, J=8.1Hz, 1H, 6-H), 7.66 (dd, J=7.2, 2.3Hz, 2H, aryl-), 7.44 (dd, J=5.1, 1.8Hz, 3H, aryl -), 5.81(dd, J=5.2, 2.9Hz, 2H, 5-H, 1'-H), 4.26(d, J=2.4Hz, 1H, 3'-H), 4.23(d, J=3.6 Hz, 1H, 2'-H), 3.79(d, J=18.4Hz, 1H, 5'-H), 3.30(d, J=18.4Hz, 1H, 5'-H), 0.95(s, 9H) , 0.88(s, 9H), 0.12(t, J=15.8Hz, 12H); 13 C NMR (75MHz, CDCl 3 )δ163.67, 158...
Embodiment 3
[0055] Example 3: Synthesis
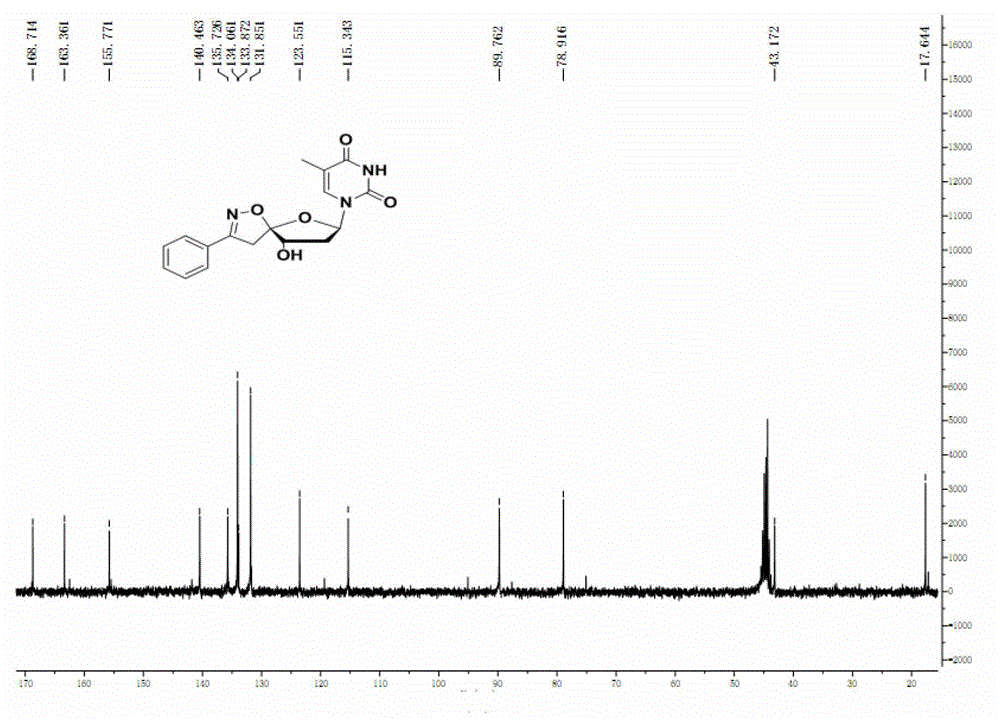
[0056] Weigh 1mmol of the cyclization product and dissolve it in 5ml of anhydrous THF to dissolve it, then add 1NTBAF (tetrabutylammonium fluoride, 3ml) tetrahydrofuran solution, stir at room temperature for 3h, after the reaction is completed, pour the reaction system into 10ml of ice water, a white solid will precipitate out at this time. The white solid was filtered and washed with water, ethanol and methanol successively. The pure product was obtained. Two-step yield: 62%, 1 H NMR (300MHz, DMSO-d 6 )δ=7.70(t, J=8.0Hz, 3H), 7.55-7.40(m, 3H), 6.10(dd, J=9.6, 5.8Hz, 2H), 5.82(d, J=8.3Hz, 1H) , 5.79(d, J=6.2Hz, 1H,), 4.34(d, J=4.1Hz, 1H,), 4.08(t, J=4.5Hz, 1H), 3.81(d, J=18.9Hz, 1H) , 3.45 (d, J=18.8Hz, 1H). 13 C NMR (75MHz, DMSO-d 6 )δ=163.00, 158.03, 150.95, 139.91, 130.70, 128.97, 128.57, 126.80, 117.00, 103.04, 87.40, 73.86, 73.79, 40.70.
[0057] Calculated value of high resolution mass spectrum: C 16 h 16 N 3 o 6 [M+H] + : 346...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com