Preparation method of lithium iron phosphate anode material co-coated by conducting polymer/nanometer metal particles
A technology of nano-metal particles and conductive polymers, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of loss of electrical conductivity, achieve good electronic conductivity, improve weak electrical conductivity, and maintain cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
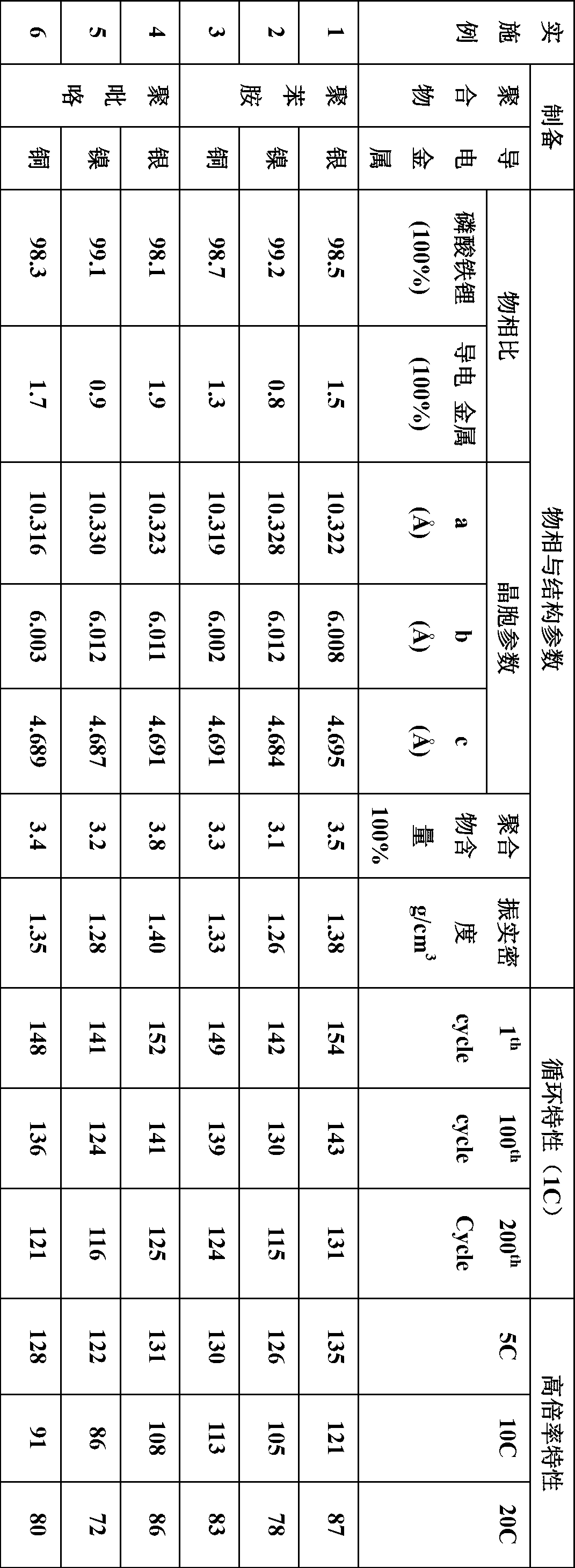
[0024] Dissolve 0.11 g of aniline in 20.0 mL of 0.4 mol / L formic acid solution, stir and disperse for 30 min, then weigh 4.08 g of lithium iron phosphate powder, stir and disperse in the above solution and ultrasonically disperse for 2 h. After that, 0.1287 g of silver nitrate was weighed, poured into the mixed solution, and the reaction mixture was ultrasonically dispersed for 10 min to dissolve. Then weigh 0.27g of ammonium persulfate as the oxidant ammonium persulfate according to the 1:1 molar ratio of oxidant and polymer monomer, pour it into a flask, and keep the reaction mixture in an ultrasonic water bath at 0°C for 4h, cool to room temperature, and let it stand for 2h , and then filtered and washed three to five times with corresponding concentrations of acid solution and acetone, and vacuum dried at 80°C for 12 hours to obtain a high-rate lithium iron phosphate cathode material.
[0025] Take the active material polymer-coated lithium iron phosphate powder, acetylene...
Embodiment 2
[0028] Dissolve 0.11 g of aniline in 20.0 mL of 0.4 mol / L formic acid solution, stir and disperse for 30 min, then weigh 4.08 g of lithium iron phosphate powder, stir and disperse in the above solution and ultrasonically disperse for 2 h. Take by weighing 0.1287g nickel nitrate Ni (NO 3 ) 2 ·6H 2 O, poured into the mixed solution, and the reaction mixture was ultrasonically dispersed for 10 min to dissolve. Then weigh 0.27g of ammonium persulfate as the oxidant ammonium persulfate according to the 1:1 molar ratio of oxidant and polymer monomer, pour it into a flask, and keep the reaction mixture in an ultrasonic water bath at 0°C for 4h, cool to room temperature, and let it stand for 2h , and then filtered and washed three to five times with corresponding concentrations of acid solution and acetone, and vacuum dried at 80°C for 12 hours to obtain a high-rate lithium iron phosphate cathode material.
[0029] Take the active material polymer-coated lithium iron phosphate powd...
Embodiment 3
[0032] Dissolve 0.11 g of aniline in 20.0 mL of 0.4 mol / L formic acid solution, stir and disperse for 30 min, then weigh 4.08 g of lithium iron phosphate powder, stir and disperse in the above solution and ultrasonically disperse for 2 h. Take by weighing 0.1287g copper nitrate Cu (NO 3 ) 2 ·3H 2O, poured into the mixed solution, and the reaction mixture was ultrasonically dispersed for 10 min to dissolve. Then weigh 0.27g of ammonium persulfate as the oxidant ammonium persulfate according to the 1:1 molar ratio of oxidant and polymer monomer, pour it into a flask, and keep the reaction mixture in an ultrasonic water bath at 0°C for 4h, cool to room temperature, and let it stand for 2h , and then filtered and washed three to five times with corresponding concentrations of acid solution and acetone, and vacuum dried at 80°C for 12 hours to obtain a high-rate lithium iron phosphate cathode material.
[0033] Take the active material polymer-coated lithium iron phosphate powde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com