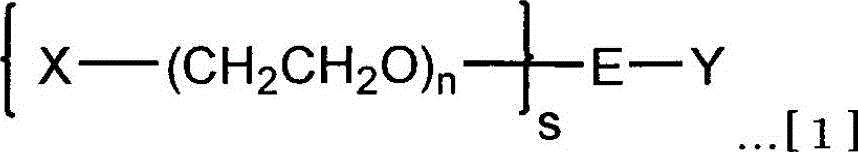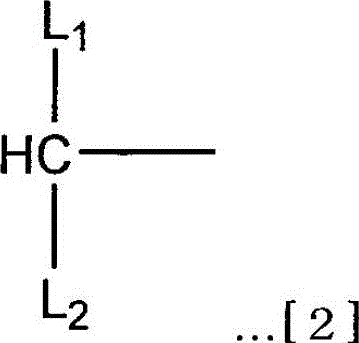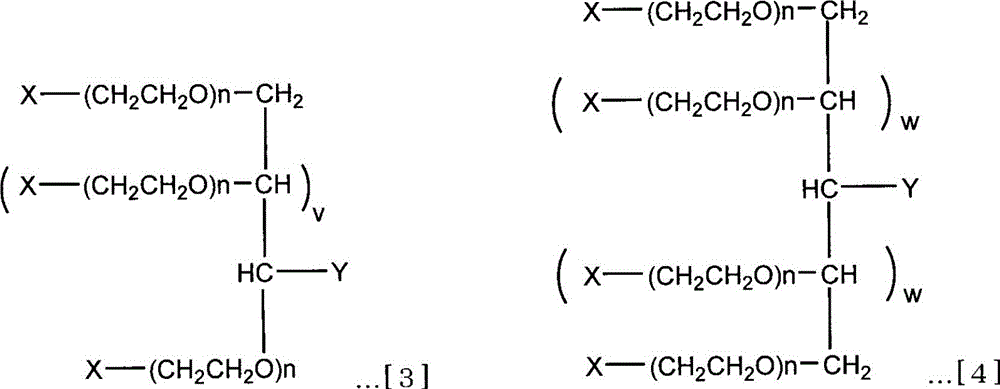Branched Isopolyethylene Glycols and Intermediates
A technology of polyethylene glycol and intermediate, applied in the field of branched heteropolyethylene glycol compounds, to achieve the effect of inhibiting the reduction of physiological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0119] Add 18.2 g (0.1 mol) of 3-benzyloxy-1,2-propanediol, 150 g of anhydrous toluene, 0.9 g (39 mmol: 26 %) sodium metal, and the whole was stirred at room temperature under nitrogen gas until the sodium metal was dissolved. The solution was charged into a 5 L autoclave, and after replacing the inside of the system with nitrogen, the temperature was raised to 100°C. After adding thereto 1,982 g (45 mol) of ethylene oxide at 100 to 130° C. and a pressure of 1 MPa or less, the reaction was further continued for 2 hours. After removing unreacted ethylene oxide gas under reduced pressure, the whole was cooled to 60°C, and the pH was adjusted to 7.5 using 85% phosphoric acid aqueous solution to obtain the following compound (a1).
[0120] 1 H-NMR; δ(ppm):
[0121] 3.40-3.90(1785H,m,HO( CH 2 CH 2 O) n - CH 2 ,HO( CH 2 CH 2 O) n - CH , CH 2 O-CH 2 Ph),4.54(2H,s,- CH 2 Ph),7.27-7.38(5H,m,-CH 2 Ph )
[0122] GPC analysis:
[0123] Number average molecular we...
Embodiment 2)
[0127] Into a 3 L four-necked flask equipped with a thermometer, a nitrogen gas inlet tube, a stirrer, a Dean-stark tube and a cooling tube, 372 g (18.6 mmol) of the above compound (a1) and 1,860 g of toluene were charged. The whole was heated under reflux and water was removed as an azeotrope. After cooling to room temperature, 6.02 g (59.5 mmol) of triethylamine and 5.54 g (48.4 mmol) of methanesulfonyl chloride were added thereto, and allowed to react at 40° C. for 3 hours. Subsequently, a solution of 85.6 g (465 mmol) of 3,3-diethoxy-1-propanol in toluene (256.8 g) containing 2.58 g (112 mmol) of sodium dissolved therein was added thereto, followed by reaction at 70°C for 5 Hour. After the reaction solution was filtered, the filtrate was transferred to a 10 L stainless steel tank, and crystallization was performed by adding 1.488 g of ethyl acetate, 1,488 g of ethanol, and 2,976 g of hexane. After the precipitated crystals were filtered to remove the solvent, the crystal...
Embodiment 3)
[0135] 50 g of the above-mentioned compound (a2) and 25 g of 5% palladium-carbon (50% hydrated product) were charged in a 1 L four-necked flask equipped with a thermometer, a nitrogen inlet tube, a stirrer and a cooling tube. After replacing with nitrogen, 400 g of methanol and 67 g of cyclohexene were added thereto, the temperature was raised, and gentle reflux was performed at 52 to 55° C. to carry out a reaction for 2 hours. After cooling the reaction solution to room temperature, palladium-carbon was filtered off, and the filtrate was concentrated. To the concentrate, 400 g of toluene and 200 g of hexane were added, and crystallization was performed. The resulting crystals were collected by filtration and dried to obtain the following compound (a3).
[0136] 1 H-NMR; δ(ppm):
[0137] 1.16-1.24(12H,t,( CH 3 CH 2 O) 2 -CHCH 2 CH 2 -),1.85-1.95(4H,q,(CH 3 CH 2 O) 2 -CH CH 2 CH 2 -),3.40-3.90(1673H,m,-( CH 2 CH 2 O) n - CH 2 ,-( CH 2 CH 2 O) n - CH , ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com