Oxaliplatin folic acid targeted lipidosome and application thereof
A technology for targeting liposomes and oxaliplatin, which is applied in the directions of liposome delivery, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., which can solve the problem of high process requirements and low encapsulation efficiency. , expensive raw materials and other problems, to achieve the effect of simple process, high encapsulation rate, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of folic acid coupling polyethylene glycol monostearate
[0041] Precisely weigh 0.5 g of folic acid, 0.5 mL of triethylamine, and 15 mL of DMSO to dissolve, add 0.6 g of dicyclohexylcarbodiimide (DCC), 0.26 g of N-hydroxysuccinimide (NHS), and stir at room temperature in the dark. Overnight, by-products were removed by filtration. Then add 5g of polyethylene glycol (molecular weight: 4000) monostearate, heat to 35°C to dissolve, and react overnight at 35°C to obtain a crude product. Pour it into a dialysis bag with a molecular cut-off of 3500, place it in a 500 mL beaker filled with water, stir, dialyze for two days, and freeze-dry to obtain it.
[0042] The synthetic route map is as follows:
[0043]
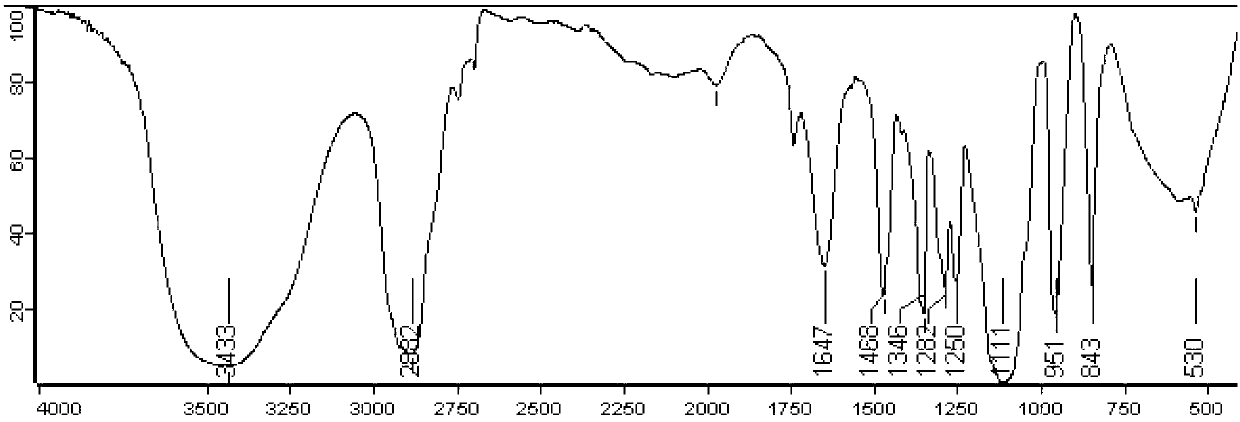
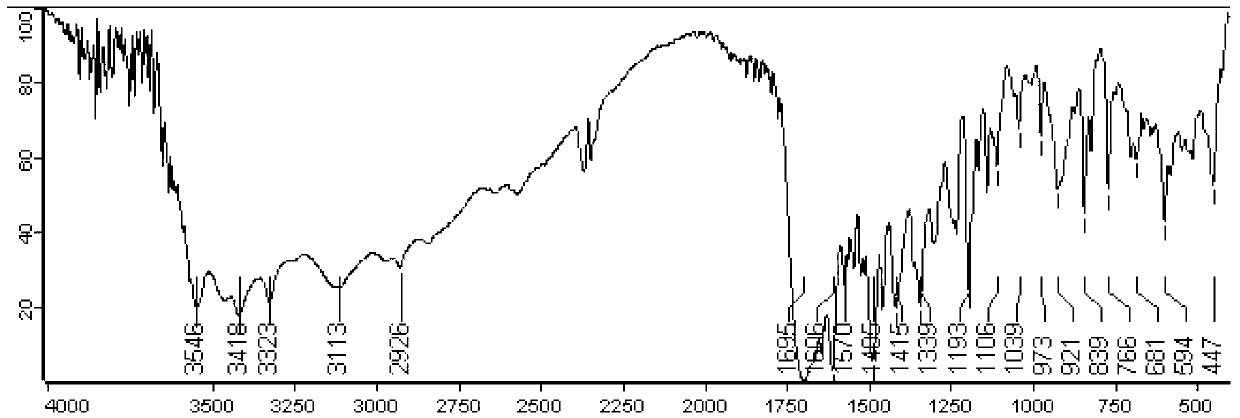
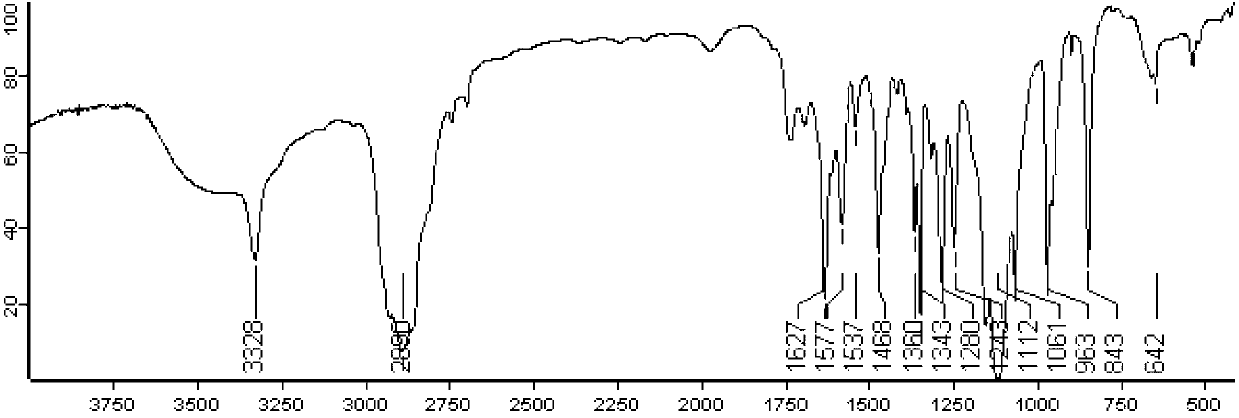
[0044] After synthesis, the infrared spectrum and hydrogen nuclear magnetic resonance spectrum of folic acid-coupled polyethylene glycol monostearate were investigated to study its structure. figure 1 It is the red absorption spectrum of ...
Embodiment 2
[0045] Example 2 Oxaliplatin folic acid targeting liposome 1
[0046] Direct preparation method: Dissolve 4 g of lecithin and 667 mg of cholesterol in 60 mL of dichloromethane, add 20 mL of an aqueous solution containing 80 mg of oxaliplatin, stir for 30 min, sonicate for 20 min, and remove organic matter by rotary evaporation at 40°C. Solvent, after the gel collapses, add 50 mL of an aqueous solution containing 580 mg of folic acid-polyethylene glycol monostearate and 400 mg of F68 according to the components, continue to evaporate for 30 min, homogenize under high pressure at 400 bar for 5 min, and dilute to volume with water to 100 mL, then tangential flow ultrafiltration was used to separate free oxaliplatin, ultrafiltration was performed with a tangential flow membrane bag with a molecular cut-off of 10K, water was used as the replacement liquid, ultrafiltration was repeated three times, and finally concentrated to about 1 / 4 of the original volume, the encapsulation effic...
Embodiment 3
[0047] Embodiment 3: Oxaliplatin folic acid targeting liposome 2
[0048] Direct preparation method: Dissolve 4 g of lecithin and 667 mg of cholesterol in 60 mL of dichloromethane, add 20 mL of an aqueous solution containing 80 mg of oxaliplatin, stir for 30 min, sonicate for 20 min, and remove organic matter by rotary evaporation at 40°C. Solvent, after the gel collapses, add 580 mg of folic acid-polyethylene glycol monostearate and 50 mL of aqueous solution of F68 400 mg according to the components, continue to evaporate for 30 min, homogenize under high pressure at 400 bar for 5 min, and dilute with water to 100 mL, and then use tangential flow ultrafiltration to separate free oxaliplatin, perform ultrafiltration with a tangential flow membrane bag with a molecular cutoff of 5K, use water as the replacement liquid, repeat ultrafiltration 3 times, and finally concentrate to about original 1 / 4 of the volume, the encapsulation efficiency was measured by ultrafiltration centrif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com