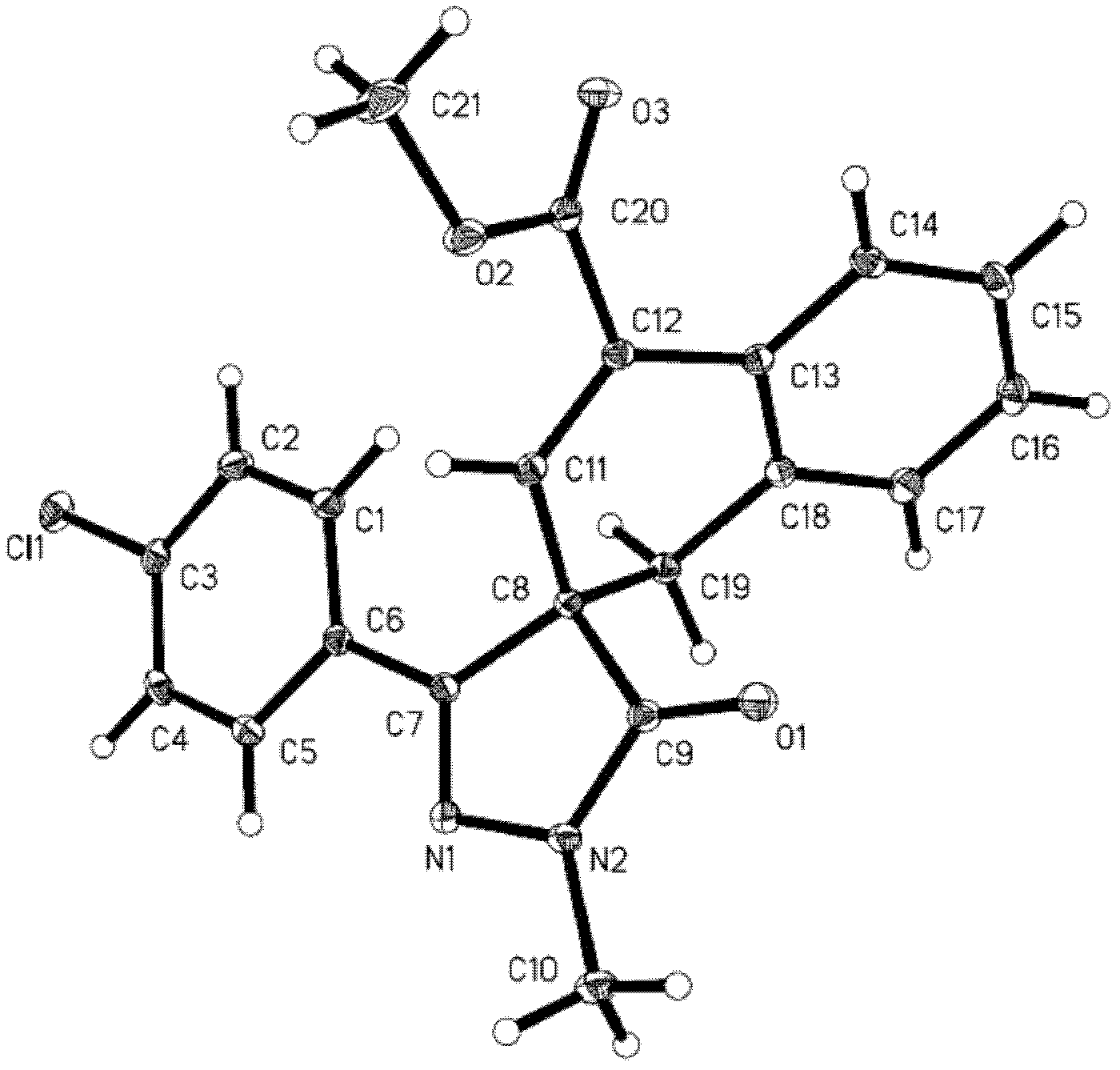
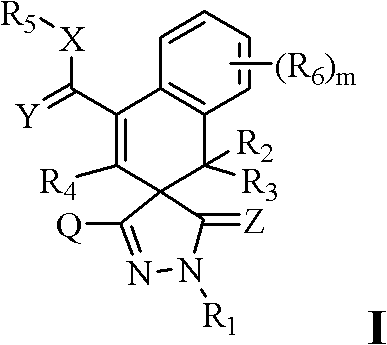
Substituted pyrazole (sulphur) ketone compound and application thereof
A technology of ketone compounds and pyrazoles, which is applied in the fields of agricultural sterilization and pesticides, and can solve the problems that have not been reported in the literature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] Embodiment 1: the preparation of compound I-A-1
[0186]
[0187] Add pyrazolol (II-1, see CN 1906171A for preparation method) (6.3 grams, 30 mmoles), benzyl chloride (III-1) (7.3 grams, 30 mmoles), anhydrous potassium carbonate (6.5 g, 47 mmol) and 40 ml of dimethyl sulfoxide, heated to 90°C with stirring, kept the reaction for 6 hours, and removed most of the solvent under reduced pressure. The residue was poured into 100 ml of water, extracted with ethyl acetate (100 ml × 3), the organic layers were combined, washed with saturated aqueous sodium chloride (100 ml), dried with anhydrous magnesium sulfate, filtered, and desolvated under reduced pressure , column chromatography (ethyl acetate:petroleum ether=1:10) of the residue gave 3.3 g of white solid (compound I-A-1), yield 28.9%, melting point 216-218°C. NMR data 1H-NMR (300MHz, internal standard TMS, solvent CDCl3) is as follows:
[0188] δ (ppm): 3.16 (d, J=16.5, 1H), 3.35 (d, J=16.5, 1H), 3.41 (s, NCH3, 3H),...
Embodiment 2
[0190] Embodiment 2: the preparation of compound I-A-2
[0191]
[0192] Compound I-A-1 (3.8 g, 10 mmol), 20 ml of ethanol and 10 ml of 10% sodium hydroxide aqueous solution were sequentially added into the reaction flask, and the temperature was raised to reflux for reaction, and the reaction was monitored by TLC. Ethanol was removed under reduced pressure, 20 ml of water was added, and the pH value was adjusted to weak acidity with dilute hydrochloric acid, and 3 g of white solid (compound I-A-2) was obtained by filtration, with a yield of 81.8% and a melting point of 200-202°C.
Embodiment 3
[0193] Embodiment 3: the preparation of compound I-A-23
[0194]
[0195] Add compound I-A-2 (0.74 g, 2 mmol) and 20 ml of tetrahydrofuran successively to the reaction flask, slowly add carbonyldiimidazole (0.35 g, 2.2 mmol) under stirring, react at room temperature for 1 hour, slowly add 1 Milliliter of 25% methylamine aqueous solution was added, and the reaction was continued for 1 hour, and the reaction was monitored by TLC. The solvent was removed under reduced pressure, and the residue was subjected to column chromatography to obtain 0.3 g of a white solid with a yield of 39.5% and a melting point of 128-130°C. NMR data 1 H-NMR (300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0196]δ(ppm): 2.78(d, J=5.1, NHCH 3 , 3H), 3.03(d, J=16.5, 1H), 3.36(s, NCH 3 , 3H), 3.43(d, J=16.5, 1H), 5.91(s, CH, 1H), 6.42(s, NH, 1H), 7.04(m, 1H), 7.27(m, 4H), 7.46(m , 2H), 7.70 (m, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com