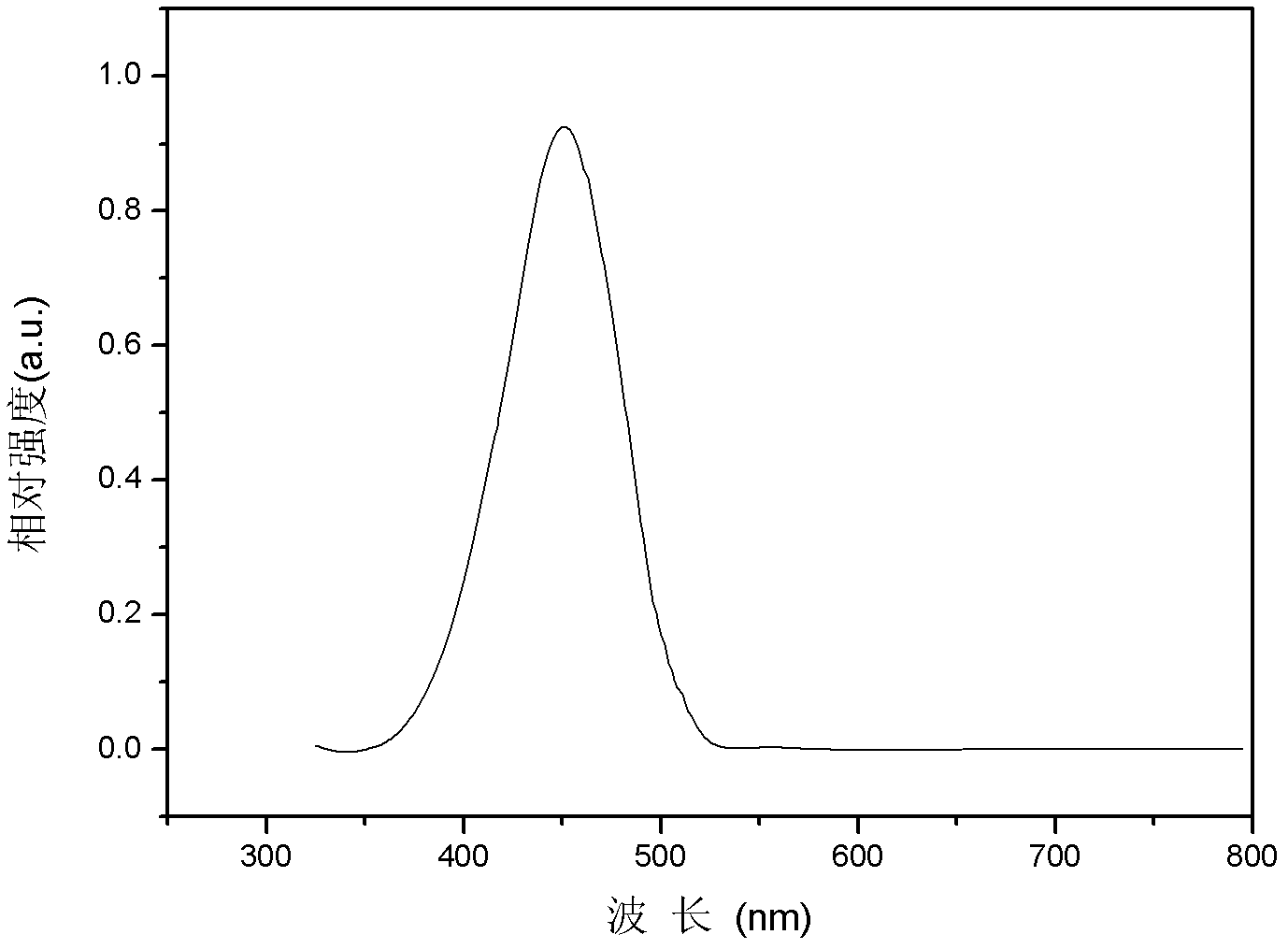
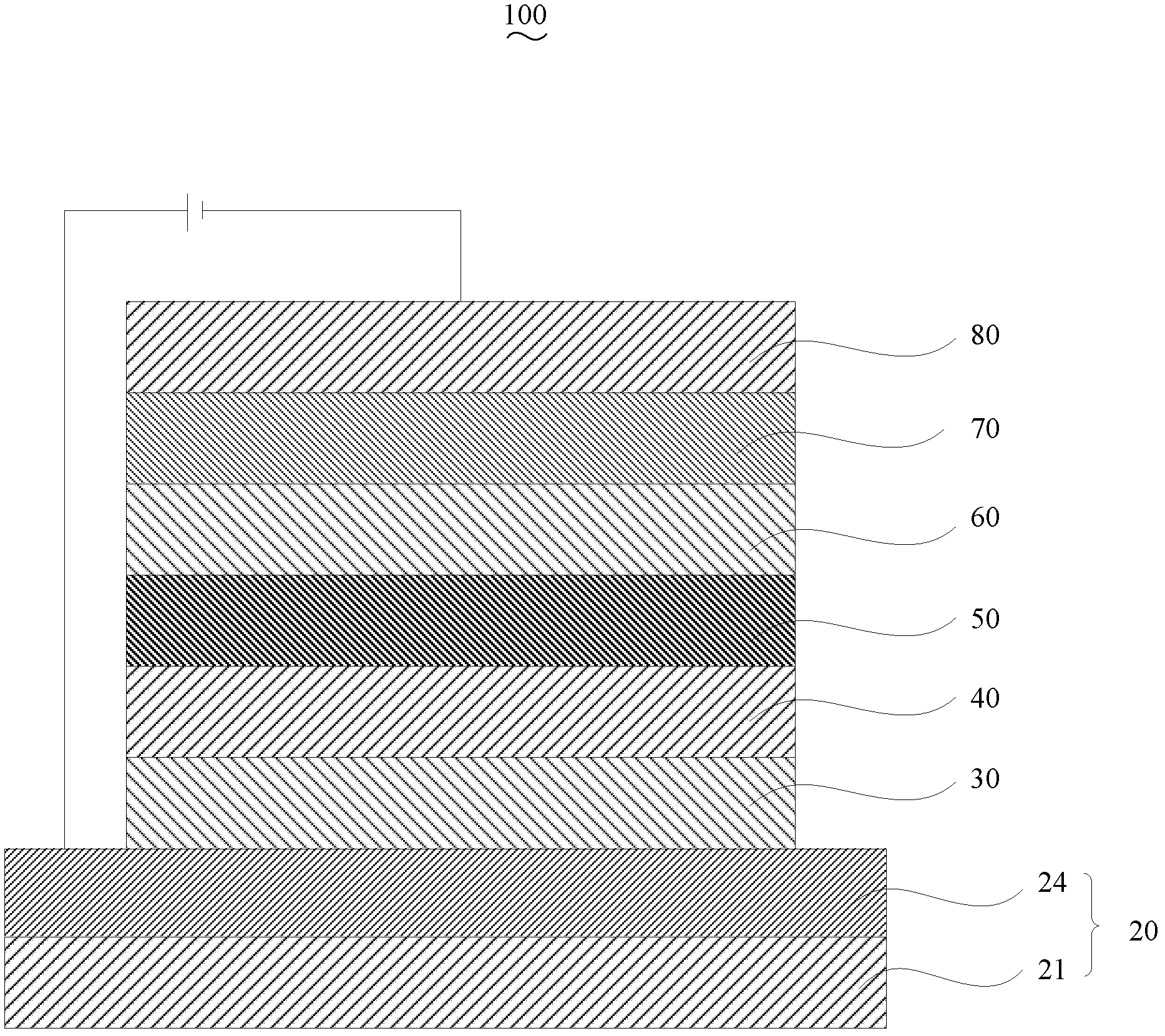
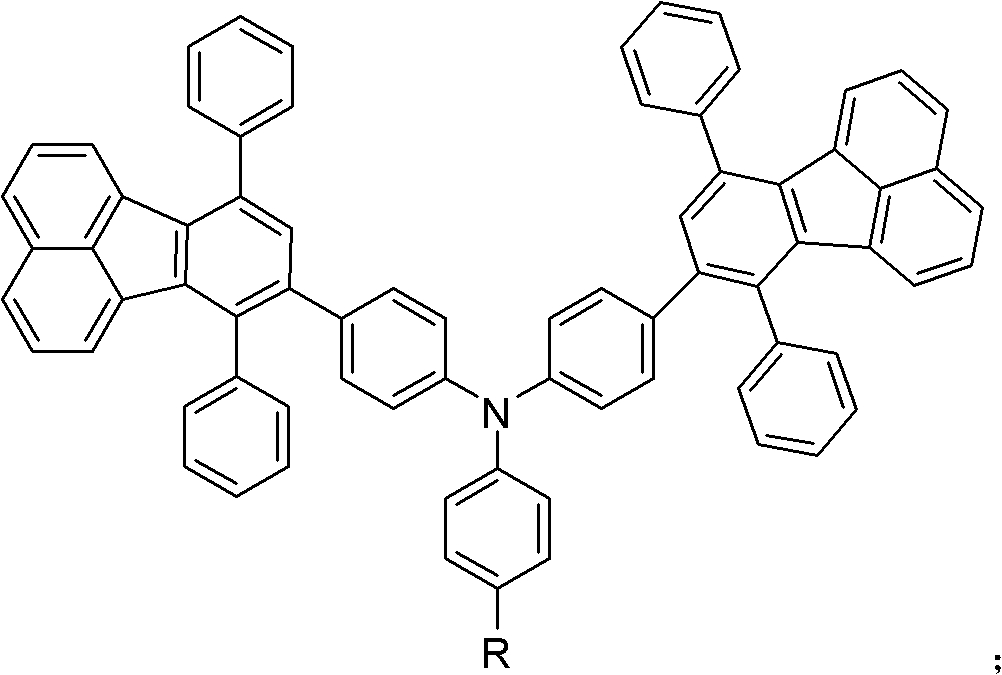
Double-fluoranthene organic semiconductor material as well as preparation method and application thereof
An organic semiconductor, double fluoranthene technology, applied in the field of double fluoranthene organic semiconductor materials and their preparation, can solve the problems of instability, high emission energy, low thermal stability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of the bisfluoranthene organic semiconductor material of one embodiment comprises the following steps:
[0043] Step S1, providing compound A and compound B (7,9-diphenyl-cyclopentenen-8-one) represented by the following structural formula:
[0044]
[0045] Among them, -R is -CN, -NO 2 、-CF 3 , -F or -CHO,
[0046]
[0047] Wherein, the preparation process of compound A comprises the following steps:
[0048] First, compound D represented by the following structural formula is provided,
[0049]
[0050] Among them, -R is -CN, -NO 2 、-CF 3 , -F, or -CHO.
[0051] Secondly, under an inert gas atmosphere, compound D and trimethylsilylacetylene are added to the solvent in a molar ratio of 1:3 to 1:3.2, and after adding the catalyst, react at reflux temperature and purify to obtain compound A. The catalyst is organic palladium Cocatalyst with cuprous iodide.
[0052] In this embodiment, the molar ratio of organic palladium and cuprou...
Embodiment 1
[0067] This example prepares 4,4'-(7,10-diphenylfluoranthenyl)-4"-cyanotriphenylamine (D2PFCTPA)
[0068] Include the following steps:
[0069] Step S11, preparing 4,4'-trimethylsilylacetylene-4"-cyanotriphenylamine (compound A).
[0070] In a three-necked flask with nitrogen, add the catalyst Pd(PPh 3 ) 2 Cl 2 (630mg, 0.9mmol), CuI (45mg, 0.45mmol), 4,4'-bromo-4"-cyanotriphenylamine (6.39g, 15mmol), trimethylsilylacetylene (4.42g, 45mmol), in 60mL Triethylamine was used as a solvent, and nitrogen gas was evacuated three times, and the oil bath was heated and refluxed for 48h. The reaction solution was washed with ether and filtered, the solvent was spin-off, and n-hexane was used as the eluent for silica gel column chromatography to obtain a light yellow solid (5.41g, Yield 78%).
[0071] The reaction formula is as follows:
[0072]
[0073] 4,4'-trimethylsilylacetylene-4"-cyanotriphenylamine H NMR test results are as follows:
[0074] 1 H NMR (400MHz, CD 2 Cl 2 ...
Embodiment 2
[0091] This example prepares 4,4'-(7,10-diphenylfluoranthenyl)-4"-trifluoromethyltriphenylamine (D2PFTFTPA)
[0092] Include the following steps:
[0093] Step S21. Preparation of 4,4'-trimethylsilylacetylene-4"-trifluoromethyltriphenylamine (compound A).
[0094] Into a three-necked flask with argon gas, add catalyst Pd(PPh 3 ) 4 (1038mg, 0.9mmol), CuI (45mg, 0.45mmol), 4,4'-bromo-4"-trifluoromethyltriphenylamine (7.06g, 15mmol), trimethylsilylacetylene (4.42g, 45mmol), Using 60mL triethylamine as solvent, vacuum three times with nitrogen gas, heat and reflux in an oil bath for 48h, wash with ether and filter, spin off the solvent, use n-hexane as eluent for silica gel column chromatography, and obtain a light yellow solid (6.06g, produced rate 80%).
[0095] The reaction formula is as follows:
[0096]
[0097] 4,4'-trimethylsilylacetylene-4"-trifluoromethyltriphenylamine proton nuclear magnetic resonance spectrum test results are as follows:
[0098] 1 H NMR (400M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Luminous efficiency | aaaaa | aaaaa |
| Brightness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com