Azithromycin dispersible tablet, as well as preparation method and application thereof
A technology of azithromycin and dispersible tablets, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of hidden dangers of drug safety, unqualified friability, poor compressibility, etc. Achieving safe selection, avoiding degradation, and good performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation method of embodiment 1 azithromycin dispersible tablet of the present invention
[0038] [prescription]
[0039]
[0040] Preparation:
[0041] 1. Mix azithromycin and micropowder silica gel through an 80-mesh sieve, and mix evenly;
[0042] 2. Mix lactose, microcrystalline cellulose, low-substituted hydroxypropyl cellulose and aspartame evenly;
[0043] 3. Pass the above two powders through a 60-mesh sieve, and after mixing evenly, add magnesium stearate and mix in a mixer for 5-10 minutes;
[0044] 4. Adjust the pressure and tablet weight of the tablet press, take the above mixed powder and perform tablet compression to obtain 1000 dispersible tablets.
[0045] In addition to using aspartame as a flavoring agent, the present invention can also use sucrose, stevioside, sodium saccharin, sucralose, edible essence and their composition.
Embodiment 2
[0046] The preparation method of embodiment 2 azithromycin dispersible tablets of the present invention
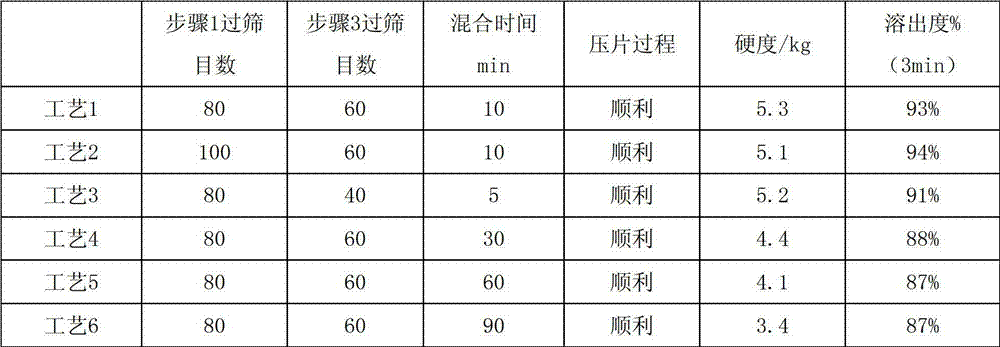
[0047] Prepare dispersible tablets according to the prescription and preparation method of Example 1, wherein the sieve mesh numbers of steps 1 and 3 and the final mixing time of step 3 refer to the following table, and the obtained dispersible tablet results are as follows:
[0048] Table 1
[0049]
[0050] From the above results, it can be seen that when the mixing time reaches 30 minutes, the hardness of the tablet drops significantly. In order to ensure the consistency of product quality, the present invention may preferably have a mixing time of 5-30 minutes.
Embodiment 3
[0051] The preferred of embodiment 3 azithromycin dispersible tablet prescription of the present invention
[0052] Due to the poor compressibility and viscosity of azithromycin itself, it is easy to cause problems such as sticking, splitting, and unqualified friability during the tableting process. This phenomenon is more serious when using the powder direct tableting process. for highlighting.
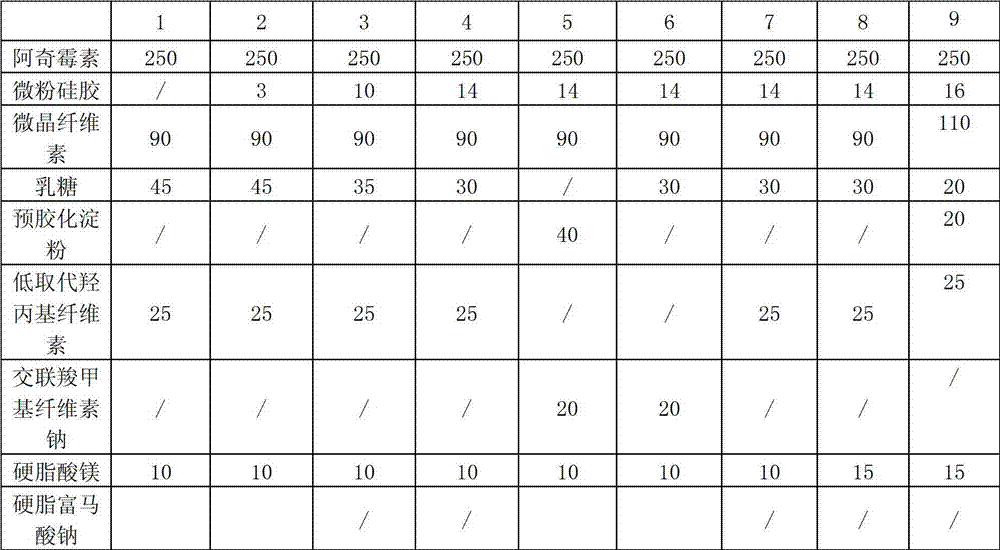
[0053] In order to obtain high-quality azithromycin dispersible tablets, the present invention screened the prescription of dispersible tablets, wherein the prescription ratio and mixing time were in accordance with Table 2, and the preparation method was referred to Example 1.
[0054] Table 2
[0055]
[0056]
[0057] Table 2 continued
[0058]
[0059]
[0060] summary:
[0061] (1) From the comparison of prescriptions 1-13, it can be seen that if micropowder silica gel is not added to the prescription, the sticking phenomenon of the dispersible tablet is more obvi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com