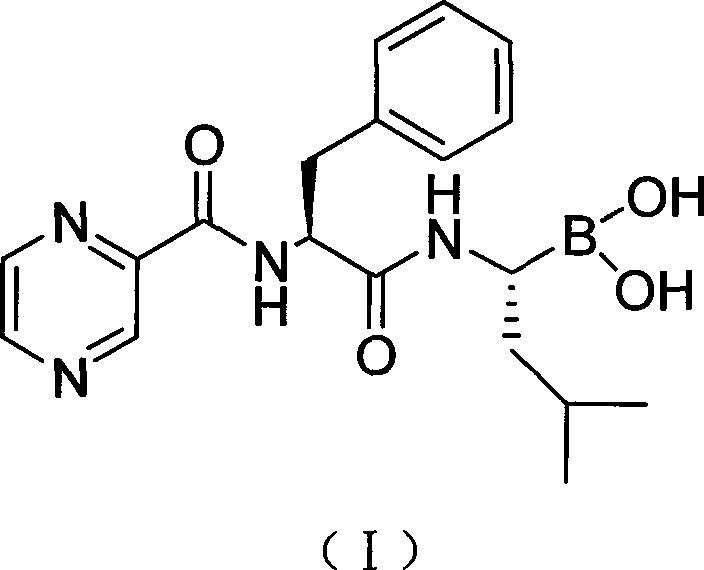
Preparation method of N-(2-pyrazine carbonyl)-L-phenylalanine-L- leucine boracic acid
A technology of leucine boric acid and phenylalanine, which is applied in the direction of chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, bulk chemical production, etc., can solve the inconvenience of large-scale industrial production, bortezomib Purity reduction, yield reduction and other problems, to achieve the effect of improving product purity and recrystallization yield, reducing degradation loss, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
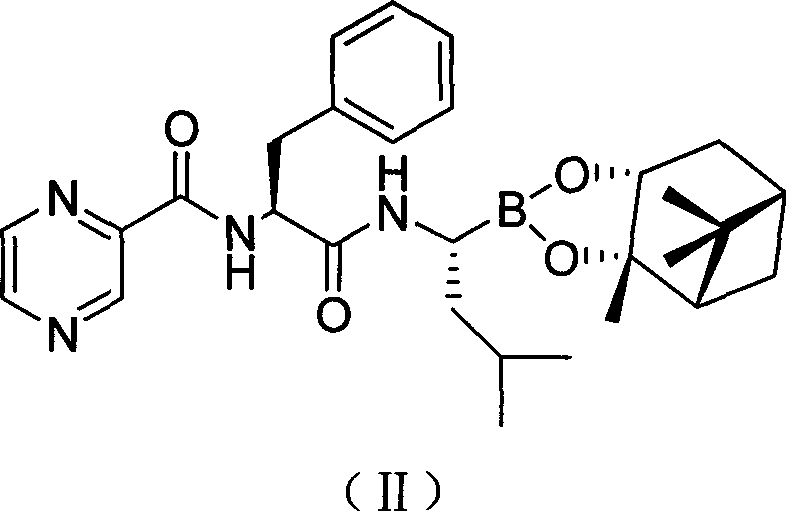
[0032] The preparation of embodiment 1 bortezomib pinanediol ester (compound II)
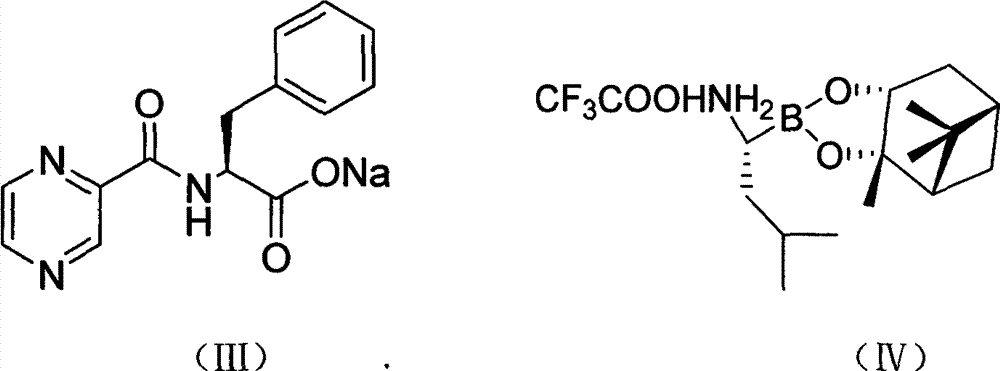
[0033] Add 7.7g of compound (III) and 9.3g of O-benzotriazole-N,N,N',N'-tetramethyluronium tetrafluoroborate (TBTU) successively into a clean three-necked flask, 300mL di Chloromethane was placed in a low-temperature reactor, mechanically stirred, protected by nitrogen, and cooled to below 0°C, and 10 g of compound (IV) was slowly added in batches. Maintain the reaction temperature for 2h, adjust the reaction temperature to 5°C, and react for 12h. Filtrate at room temperature, concentrate the filtrate to dryness under reduced pressure, add 500 mL of ethyl acetate to dissolve, wash the organic layer three times with 100 mL of water, three times with 100 mL of 1% phosphoric acid, three times with 100 mL of 2% sodium carbonate, and 100 mL of 10% sodium chloride. Wash 3 times, then wash 3 times with 100 mL of water, separate and remove the water layer, and dry the organic layer with anhydrous magne...
Embodiment 2
[0036] The preparation of embodiment 2 bortezomib crude product
[0037] Dissolve compound II with 100ml of methanol and 100ml of n-hexane, add it to a 500mL three-necked flask, place it in a low-temperature reactor, stir the temperature in the system to below 0°C, add 50mL of 1N HCl dropwise, after the dropwise addition, add isobutylboronic acid 4.2g, reacted at -5°C for 12h. After the reaction was completed, the liquid was separated, the methanol layer was washed three times with 100 mL of n-hexane, the methanol layer was concentrated, the pH was adjusted to 11 with 2N sodium hydroxide, and the methanol layer was washed three times with 100 mL of dichloromethane. Dichloromethane (100 mL) was added to the aqueous layer, the pH was adjusted to 6 with 1N hydrochloric acid, extracted three times with 100 mL of dichloromethane, combined with anhydrous magnesium sulfate and dried. After filtration and concentration, the crude product was concentrated to obtain 8 g of white solid ...
Embodiment 3
[0040] Embodiment 3 The recrystallization of bortezomib crude product
[0041] Add 140ml of ethyl acetate into the round bottom flask, then add 0.6mL of formic acid, heat to 70°C, add 10g of 81% in batches, the purity is 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com