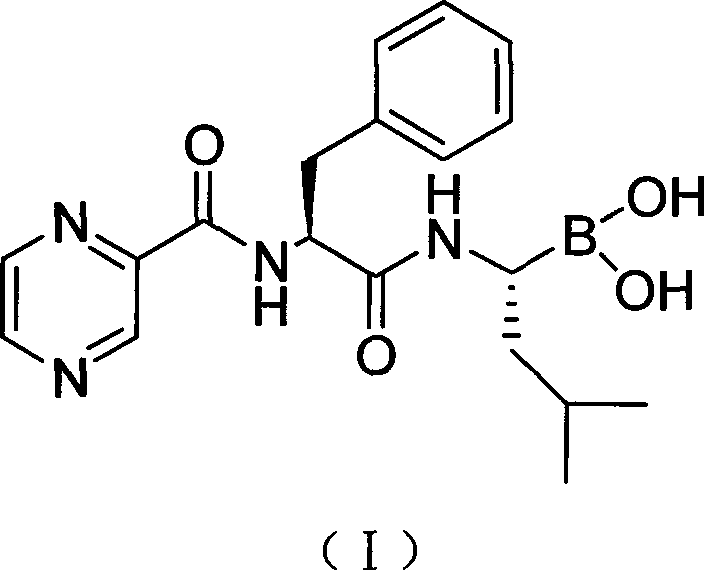
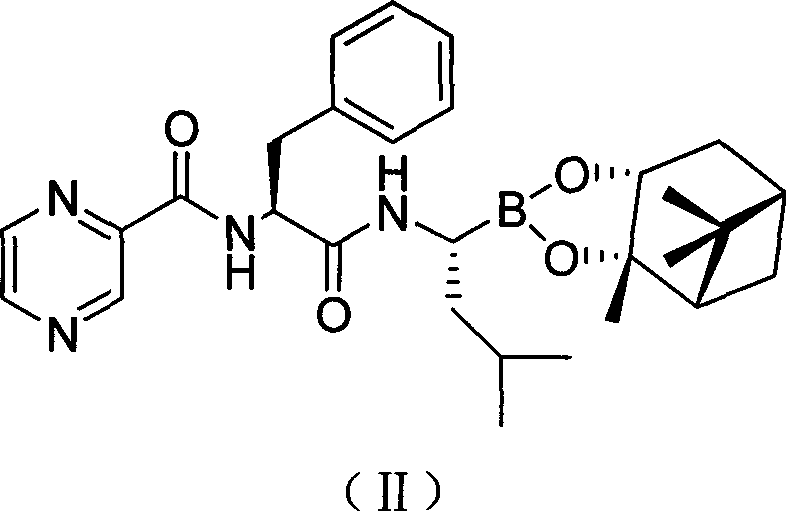
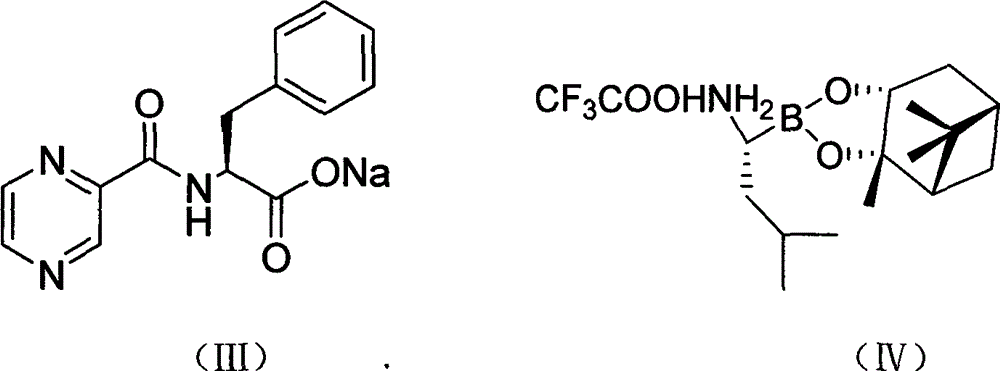
Preparation method of N-(2-pyrazine carbonyl)-L-phenylalanine-L- leucine boracic acid
A technology of leucine boronic acid and phenylalanine, applied in chemical instruments and methods, compounds containing periodic table Group 3/13 elements, production of bulk chemicals, etc., can solve the problem of bortezomib purity decrease, yield Reduce the problems of large-scale industrial production, etc., to achieve the effect of improving product purity and recrystallization yield, simple operation, and reducing degradation loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of bortezomib pinanediol ester (compound II)
[0033] In a clean three-necked bottle, 7.7g of compound (III) and 9.3g of O-benzotriazole-N,N,N',N'-tetramethylurea tetrafluoroborate (TBTU) were added successively, 300mL of two Methyl chloride, placed in a low temperature reactor, mechanically stirred, nitrogen protection, and cooled to below 0 °C, and slowly added 10 g of compound (IV) in batches. Maintain the reaction temperature for 2h, adjust the reaction temperature to 5°C, and react for 12h. Filtration at room temperature, the filtrate was concentrated to dryness under reduced pressure and dissolved in 500 mL of ethyl acetate. The organic layer was washed three times with 100 mL of water, three times with 100 mL of 1% phosphoric acid, three times with 100 mL of 2% sodium carbonate, and 100 mL of 10% sodium chloride. Washed 3 times, then washed 3 times with 100 mL of water, separated and removed the water layer, and dried the organic layer with...
Embodiment 2
[0036] The preparation of embodiment 2 bortezomib crude product
[0037] Dissolve compound II with 100ml methanol and 100ml n-hexane, add it to a 500mL three-necked flask, place it in a low temperature reactor, stir the temperature in the system to below 0°C, add 1N HCl 50mL dropwise, after the dropwise addition, add isobutylboronic acid 4.2g, react at -5°C for 12h. After the reaction was completed, the layers were separated, the methanol layer was washed three times with 100 mL of n-hexane, the methanol layer was concentrated, adjusted to pH 11 with 2N sodium hydroxide, and washed three times with 100 mL of dichloromethane. Dichloromethane (100 mL) was added to the aqueous layer, the pH was adjusted to 6 with 1N hydrochloric acid, extracted three times with 100 mL of dichloromethane, combined and dried over anhydrous magnesium sulfate. Filtration and concentration to obtain the crude product and concentrated to obtain 8 g of white solid with a yield of 91% and a purity of 98...
Embodiment 3
[0040] The recrystallization of embodiment 3 bortezomib crude product
[0041] 140 ml of ethyl acetate was added to the round-bottomed flask, 0.6 mL of formic acid was added, heated to 70° C., 10 g of 81% was added in batches, and the purity was 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com