Preparation and application of mixed structure PLGA-PLL-PEG targeting polymer carrier
A technology of polymers and intermediates, applied in the preparation of targeted nanoparticles, in the field of synthesis of poly(lactic-co-glycolic acid-polylysine-polyethylene glycol) polymers, can solve the problem of reducing body circulation time, reaction Reduced yield, many reaction steps, etc., to achieve the effects of increased in vivo stability, convenient product purification, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
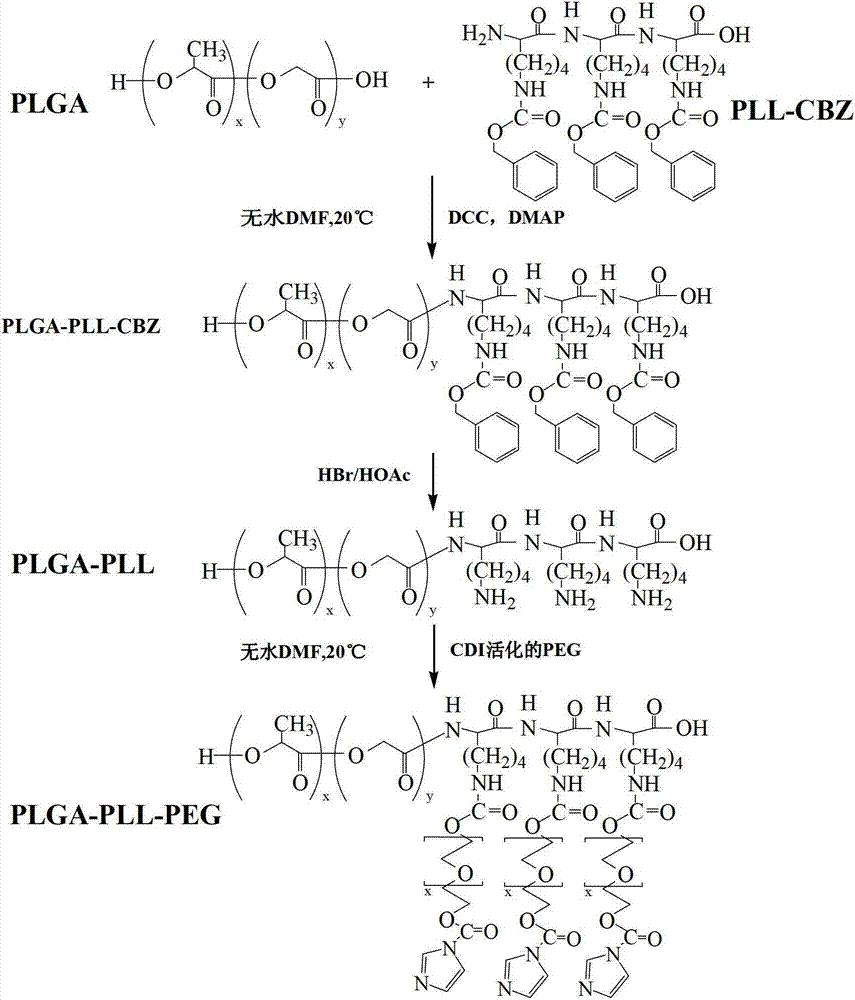
[0036] (1) Preparation of polylysine (PLL-CBZ):
[0037] Take 4g of Nε-benzyloxycarbonyl-L-lysine-N-carboxylic acid anhydride (Lys(z)-NCA) into a three-necked flask, stir and dissolve with 30ml of anhydrous DMF, protect with nitrogen, and use a micro-injector according to the molar ratio Add anhydrous ethylenediamine at a ratio of 36:1, stir at room temperature for 72 hours, add 8 times the volume of anhydrous ether to precipitate, filter with suction, and dry in vacuo overnight.
[0038] 1 H-NMR: δ=1.18-1.66(m; -(CH 2 ) 3 -CH 2 -NH-Z), δ=3.04(m; ε-CH 2 ), δ=3.78(s; α-CH), δ=4.31(s; -CO-CH-NH-), δ=5.05(s; C 6 h 5 CH 2 ; 2H), δ=6.85(s; -NH-Z; 1H), δ=7.33(m; C 6 h 5 CH 2 ; 5H), δ=7.95 (α-NH 2 ; 2H). 1 α-NH appears at δ=7.95 in the H-NMR spectrum 2 A hydrogen peak, indicating the occurrence of polymerization.
[0039] (2) Activated polyethylene glycol:
[0040] Dissolve 4g of PEG4000 in dioxane, dissolve and keep warm at 37°C, add excess CDI (molar ratio 1:8), N 2...
Embodiment 2
[0051] (1) Synthesis of polylysine (PLL-CBZ):
[0052] Take 3g of Nε-benzyloxycarbonyl-L-lysine N-carboxylic acid anhydride (Lys(z)-NCA) into a three-necked flask, stir and dissolve with 20ml of anhydrous DMF, protect with nitrogen, and use a micro-injector according to the molar ratio Add anhydrous triethylamine at a ratio of 60:1, stir for 72 hours, add 5 times the volume of anhydrous ether to precipitate, filter with suction, and dry in vacuo overnight.
[0053] (2) Synthesis of polylactic acid glycolic acid-polylysine:
[0054] Weigh quantitative PLGA-COOH and the synthesized PLL-CBZ above in a molar ratio of 1:1 and dissolve them in anhydrous DMF, add 5 times PLGA molar equivalent of DCC and 1 molar equivalent of DMAP under ice bath, N 2 Protection, reaction 36h. Trichloromethane and methanol were used to remove unreacted raw materials and by-products generated by the reaction, and the final product was vacuum-dried at room temperature for 24 hours. Take 3g of PLGA-PLL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com