Amiodarone hydrochloride injection emulsion and preparation method thereof
A technology of amiodarone hydrochloride and injection milk, which is applied in the fields of pharmaceutical formula, emulsion delivery, medical preparations containing active ingredients, etc. It can solve the problems of inability to accurately predict the distribution law, increase drug solubility, and increase toxic and side effects, etc., to achieve The preparation process is simple and easy, the effect of eliminating toxic and side effects and enhancing compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
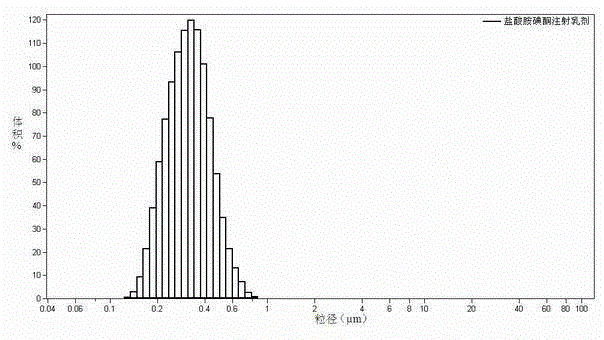
Image
Examples
Embodiment 1
[0031] prescription:
[0032] Amiodarone Hydrochloride 0.5g
[0033] soybean oil 100g
[0034] Egg yolk phospholipids 5g
[0035] Edetate Disodium 0.01g
[0036] Oleic acid 0.1g
[0037] Glycerin 20g
[0038] Sodium hydroxide solution or hydrochloric acid solution
[0039] Makes 1000ml
[0040] Preparation Process:
[0041] (1) Amiodarone hydrochloride 0.5g, soybean oil 100g, egg yolk phospholipid 5g, oleic acid 0.1g, heated in a water bath to 65°C, stirred and dissolved to obtain an oil phase;
[0042] (2) Measure 500ml of water for injection, add 20g of glycerin and 0.01g of disodium edetate, heat to 65°C and stir to dissolve to obtain the aqueous phase;
[0043] (3) Mix the oil phase and the water phase at 65°C, and shear emulsify for 15 minutes (8000 rpm), so that the oil phase and the water phase are evenly mixed to obtain colostrum;
[0044](4) Homogenize the colostrum 5 times with a high pressure homogenizer (pressure 650bar, temperature 65°C), adjust its pH to...
Embodiment 2
[0047] prescription:
[0048] Amiodarone Hydrochloride 1g
[0049] 150g peanut oil
[0050] Phosphatidylcholine 10g
[0051] 1,2-Distearoylphosphatidylglycerol 30g
[0052] Sorbitol 40g
[0053] Sodium Oleate 0.03g
[0054] Vitamin E 0.3g
[0055] Sodium hydroxide solution or hydrochloric acid solution
[0056] Make 1000ml
[0057] Preparation Process:
[0058] (1) Take 1 g of amiodarone hydrochloride and 150 g of peanut oil, heat in a water bath to 55°C, stir and dissolve to obtain an oil phase;
[0059] (2) Measure 600ml of water for injection, add 10g of phosphatidylcholine, 30g of 1,2-distearoylphosphatidylglycerol, and 40g of sorbitol, heat to 55°C and stir to dissolve to obtain the aqueous phase;
[0060] (3) Mix the oil phase and the water phase at 55°C, shear and emulsify for 15 minutes (10,000 rpm), mix the oil phase and the water phase evenly, and adjust the pH with sodium hydroxide solution (0.1mol / L) For 11.0, colostrum was obtained;
[0061] (4) Homogen...
Embodiment 3
[0065] prescription:
[0066] Amiodarone Hydrochloride 3g
[0067] Hydrogenated corn oil 200g
[0068] Egg yolk phospholipids 25g
[0069] 1,2-Distearoylphosphatidylglycerol 25g
[0070] Disodium Calcium Edetate 0.05g
[0071] Oleic acid 0.5g
[0072] Xylitol 30g
[0073] Sodium hydroxide solution or hydrochloric acid solution
[0074] Make 1000ml
[0075] Preparation Process:
[0076] (1) Take 3g of amiodarone hydrochloride, 200g of hydrogenated corn oil, 25g of egg yolk phosphatidylglycerol, 25g of 1,2-distearoylphosphatidylglycerol, 0.5g of oleic acid, heat in a water bath to 75°C, stir and dissolve to obtain the oil phase;
[0077] (2) Measure 800ml of water for injection, add 30g of xylitol, 0.05g of disodium calcium edetate, heat to 75°C and stir to dissolve to obtain the aqueous phase;
[0078] (3) Mix the oil phase and the water phase at 75°C, shear and emulsify for 20 minutes (10,000 rpm), so that the oil phase and the water phase are evenly mixed to obtain c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com