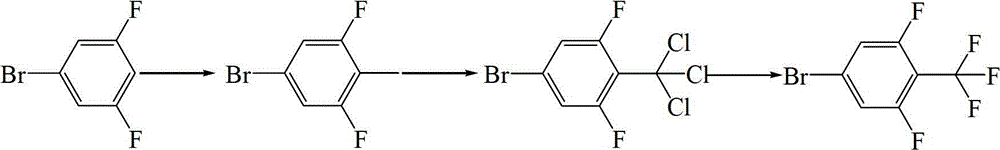
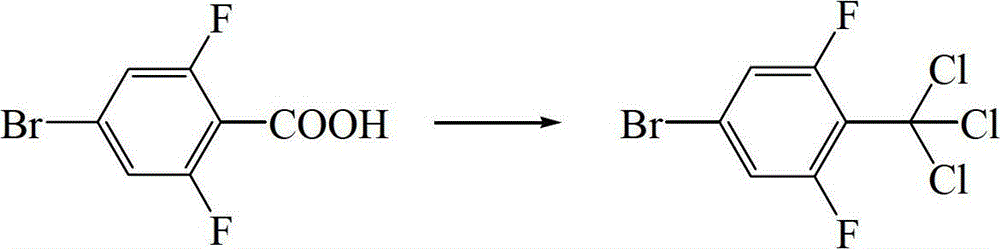Preparation methods of 4-bromo-2,6-difluoro-trifluorotoluene and intermediate thereof
A technology of difluorobenzotrifluoride and difluorobenzotrifluoride, which is applied in the field of preparation of 4-bromo-2,6-difluorobenzotrifluoride and its intermediates, can solve the problems of unsuitability for industrialized production, serious environmental pollution, Harsh reaction conditions and other problems, to achieve the effect of easy industrial production, high reaction conversion rate, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
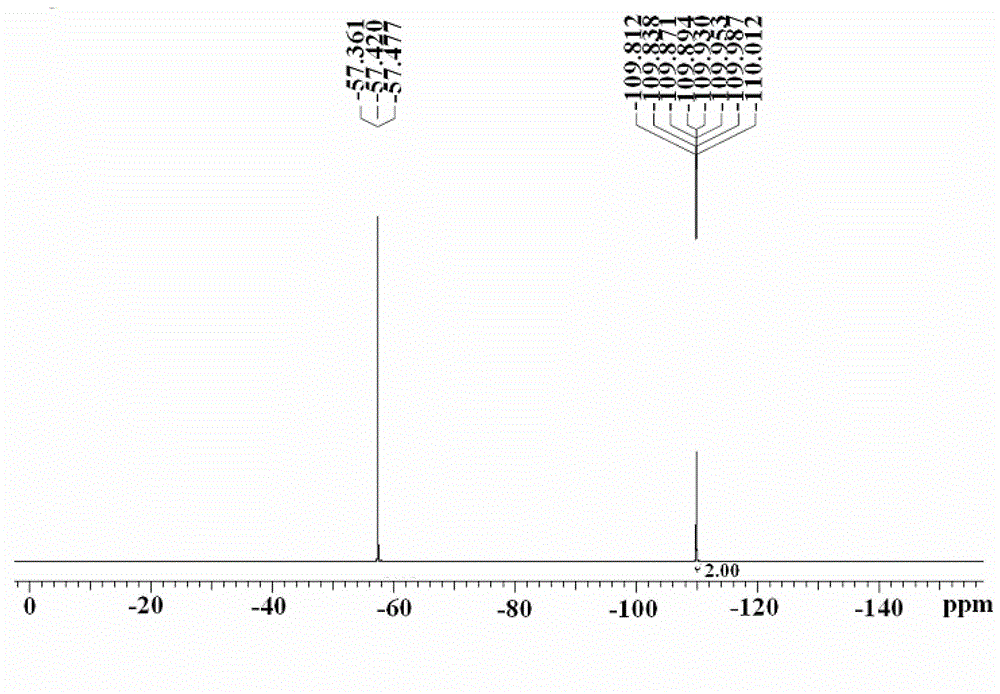
[0055] Example 1 Preparation of 4-bromo-2,6-difluorobenzotrifluoride
[0056] Step (1) Preparation of 4-bromo-2,6-difluorobenzotrichloride
[0057] 500mL four-necked bottle, install a thermometer, mechanical stirring, condenser, tail gas absorption device, under the protection of nitrogen, add 118.5g (0.5mol) 4-bromo-2,6-difluorobenzoic acid, 250.2g (1.2mol) Phosphorus chloride, 146.2g (0.75mol) phenylphosphonyl dichloride, after the addition is complete, the temperature is raised to 90~105°C, and the temperature of the reaction system is kept at 90~105°C for 9 hours. GC detects that the reaction of the raw materials is complete, and the temperature of the reaction bottle is lowered. Refit the device to vacuum distillation, distill out the by-product phosphorus oxychloride, cool down to room temperature, pour the oily residue into 5% aqueous solution of sodium bicarbonate, adjust the pH of the system to 7~8, add n-hexane for extraction, and separate layers , the aqueous layer...
Embodiment 2
[0063] Example 2 Preparation of 4-bromo-2,6-difluorobenzotrifluoride
[0064] Step (1) Preparation of 4-bromo-2,6-difluorobenzotrichloride
[0065] 500ml four-necked bottle, install a thermometer, mechanical stirring, condenser, tail gas absorption device, under the protection of nitrogen, add 118.5g (0.5mol) 4-bromo-2,6-difluorobenzoic acid, 312.7g (1.5mol) five Phosphorus chloride, 195g (1mol) phenylphosphonyl dichloride, after the addition is complete, heat up to 90~105°C, keep the temperature of the reaction system between 90~105°C for 15 hours, check that the reaction of the raw materials is complete, cool down the reaction bottle, and refit the device For vacuum distillation, distill out the by-product phosphorus oxychloride, cool down to room temperature, pour the oily residue into 5% aqueous solution of sodium bicarbonate, adjust the system pH=7~8, add n-hexane for extraction, separate layers, and water layer Back-extracted once, combined the organic layers, passed th...
Embodiment 3
[0068] Example 3 Preparation of 4-bromo-2,6-difluorobenzotrifluoride
[0069] Step (1) Preparation of 4-bromo-2,6-difluorobenzotrichloride
[0070] 500mL four-necked bottle, install a thermometer, mechanical stirring, condenser, tail gas absorption device, under the protection of nitrogen, add 118.5g (0.5mol) 4-bromo-2,6-difluorobenzoic acid, 250.2g (1.2mol) Phosphorous chloride, 146.2g (0.75mol) phenylphosphine dichloride, after the addition is completed, the temperature is raised to 90~105°C, and the temperature of the reaction system is kept at 90~105°C for 9 hours. The device is converted to vacuum distillation, the by-product phosphorus oxychloride is distilled off, the temperature is lowered to room temperature, the oily residue is poured into 5% sodium bicarbonate aqueous solution, the pH of the system is adjusted to 7~8, n-hexane is added for extraction, and the layers are separated. The aqueous layer was back-extracted once, the organic layers were combined, passed t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com