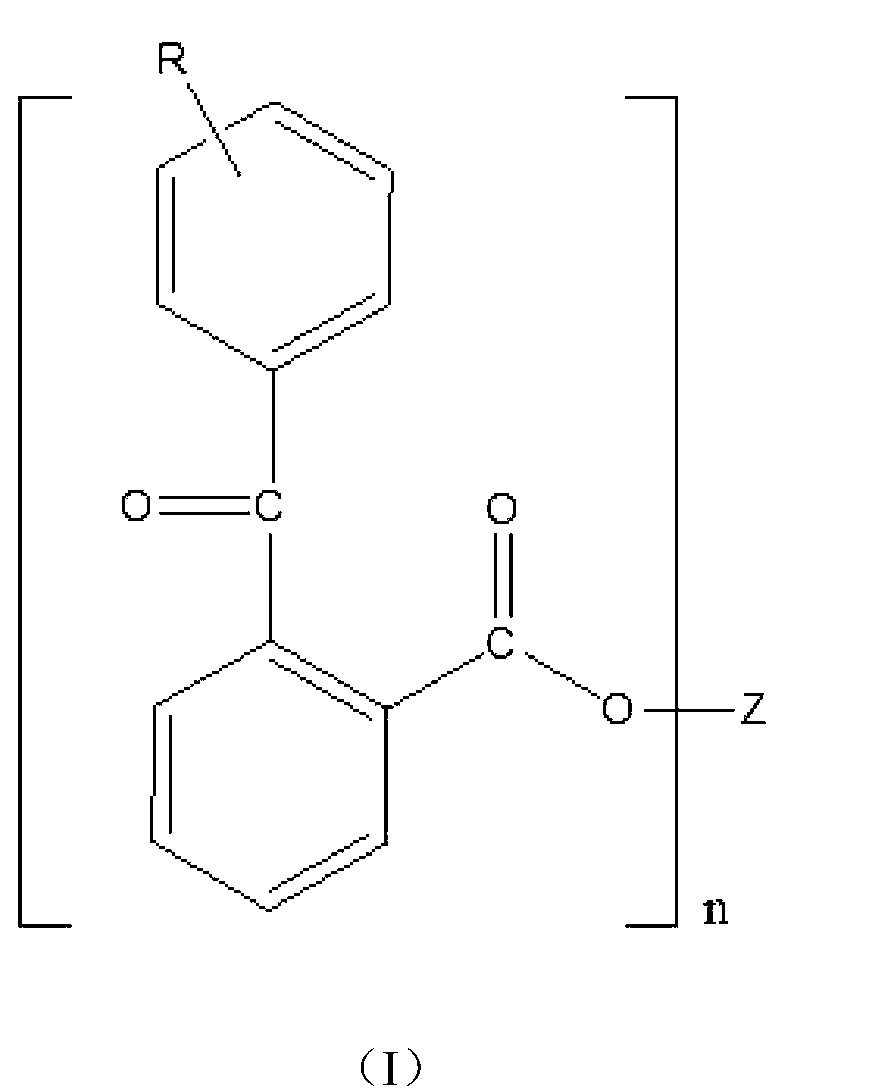
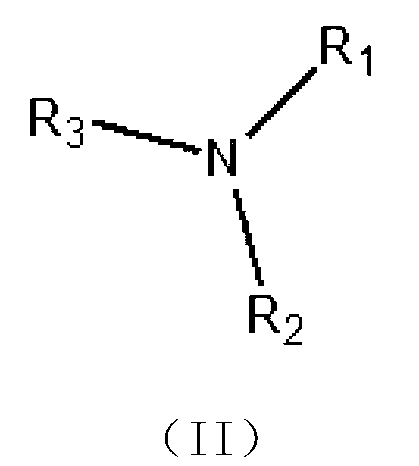
Benzophenone-type light initiator and preparation method thereof
A technology of benzophenones and photoinitiators, which is applied to the preparation of sulfides, chemical instruments and methods, and the preparation of organic compounds. Fast rate, reduced migration, improved yellowing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
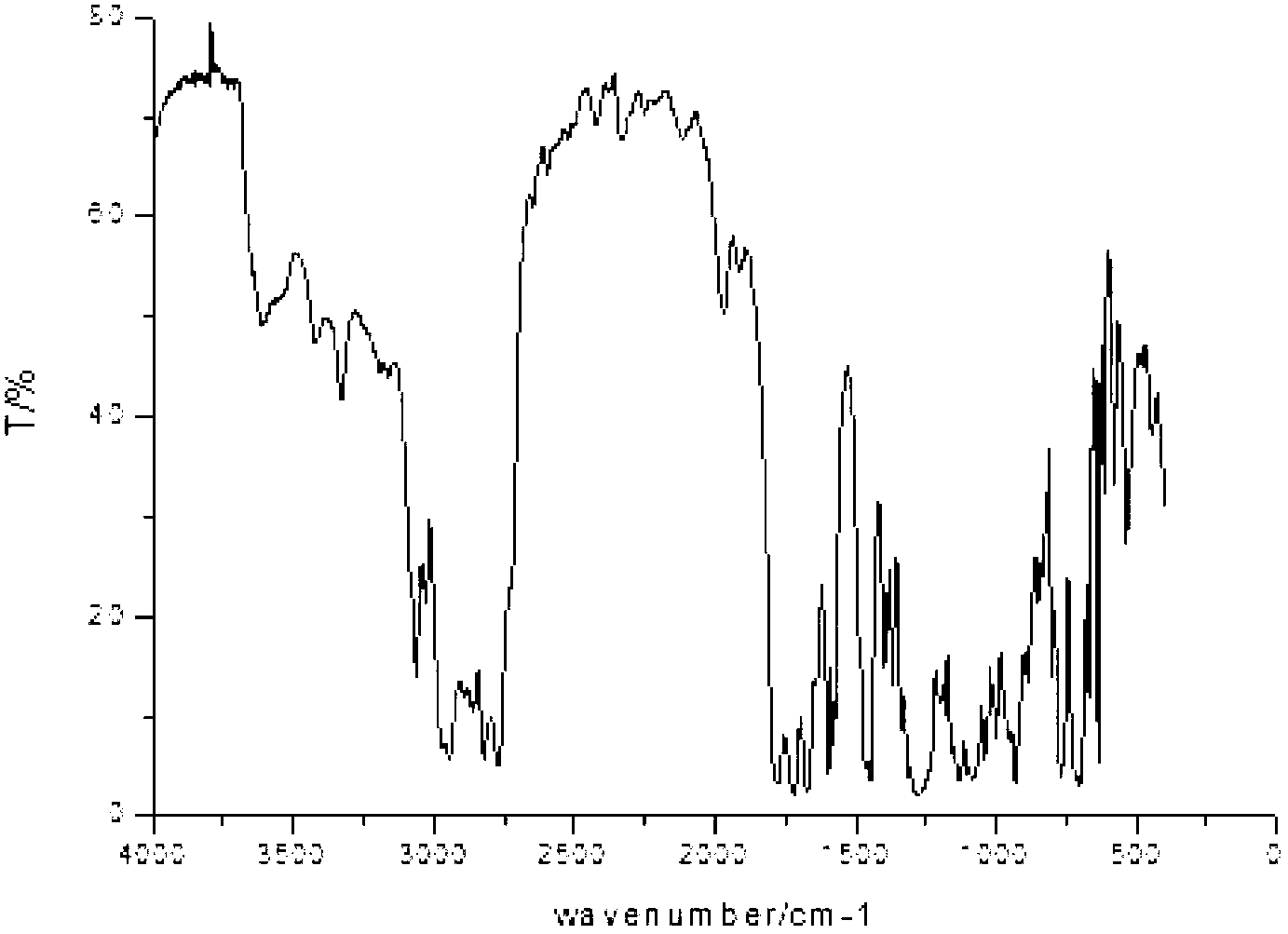
Image
Examples
Embodiment 1
[0047] Embodiment 1: the preparation of photoinitiator P6216
[0048]
[0049] a. Add 30g of phthalic anhydride and 120g of anhydrous benzene into a 75Oml three-necked flask, then heat and stir slightly on a water bath. After the phthalic anhydride is completely dissolved, slowly add 60g of anhydrous trichloride in batches Al, the reaction starts immediately. After reacting for 1.5h-2.0h, take the reaction solution for testing. When the reaction reaches the end point, slowly add 250ml of H2SO48% aqueous solution, and stir while adding, until all the 250ml of dilute H2SO4 aqueous solution is added. Then, water vapor was introduced from one side port of the three-neck round bottom flask, and the other side port was connected with a condenser tube, and excess benzene was steamed out by steam distillation. After the benzene is completely evaporated, stop heating, and when the reaction solution is cooled to 60°C to 65°C, slowly add 250ml of clear water to the reaction mixture,...
Embodiment 2
[0052] Embodiment 2: the preparation of photoinitiator P6221
[0053]
[0054] a. Add 30g of phthalic anhydride and 120g of mesitylene into a 75Oml three-necked flask, then heat and stir slightly on a water bath. After the phthalic anhydride is completely dissolved, slowly add 60g of anhydrous trichloride in batches Al, the reaction starts immediately. After reacting for 1.5h-2.0h, take the reaction solution for testing. When the reaction reaches the end, slowly add 250ml of H 2 SO48% aqueous solution, stirring while adding, until 250ml of dilute H2SO4 aqueous solution is completely added. Then, control the temperature to be 60°C and let it stand for stratification, remove the mesitylene layer in the separatory funnel, extract the lower aqueous solution with 120g of hot mesitylene once, combine the mesitylene solution, and wash twice with 100ml of water at 60°C After drying, the solvent was removed to obtain 53g of intermediate 2-(2,4,6-trimethylbenzoyl)benzoic acid, the...
Embodiment 3
[0057] Embodiment 3: the preparation of photoinitiator P6230
[0058]
[0059] a, refer to step a of the preparation of photoinitiator P6216 to prepare intermediate A o-benzoylbenzoic acid.
[0060] b. Prepare intermediate B o-benzoylbenzoyl chloride with reference to step b of the preparation of photoinitiator P6216.
[0061] c. Add 8.5ml of triethanolamine, 95ml of anhydrous benzene, and 19g of solid sodium bicarbonate in sequence in a 250ml three-necked flask with a water separator. Slowly add o-benzoylbenzoyl chloride in the step under stirring at room temperature, dropwise During the process, the temperature is controlled at 20°C-40°C. After the dropwise addition is completed, the temperature is raised to reflux for 4 hours. During the reaction, the water brought out is continuously separated. The GC monitors the reaction end point. After the completion, cool to room temperature and add 60ml of water to dissolve the salt. , standing for liquid separation, the oil phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com