Litopenaeus vannamei metallothionein gene LvMT as well as coding gene and application thereof
A technology of metallothionein and vannamene, applied in the field of genetic engineering, can solve the problems of MT gene identification and functional analysis that have not been reported, and achieve the effect of improving the ability to tolerate heavy metals, large economic value, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Cloning and sequencing of the metallothionein gene LvMT of Litopenaeus vannamei
[0028] 1. Design of primers
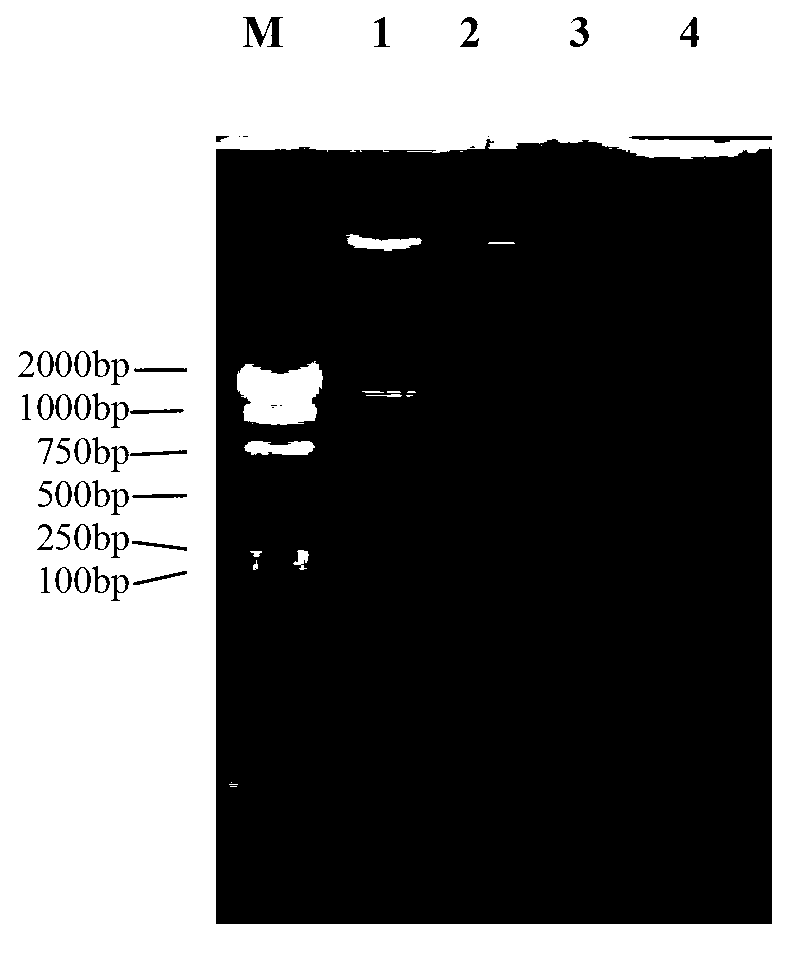
[0029] The full-length EST of the metallothionein gene of Litopenaeus vannamei was screened from the freshwater stress library of Litopenaeus vannamei, and the upstream primer (5'-ACG GGATCC GCCACCATGCCTGATCCATGCTGT-3') (the underline indicates the restriction site BamHI) and the downstream primer (5'-AGC AAGCTT CTGGGCAGCACTT-3') (the underline indicates the enzyme cutting site HindIII), and the primers were synthesized by Yingwei Jieji (Shanghai) Trading Co., Ltd.
[0030] 2. Extraction of RNA
[0031] Using the gill tissue of Litopenaeus vannamei as the material, the total RNA was extracted by the Trizol method, and the specific operation was as follows:
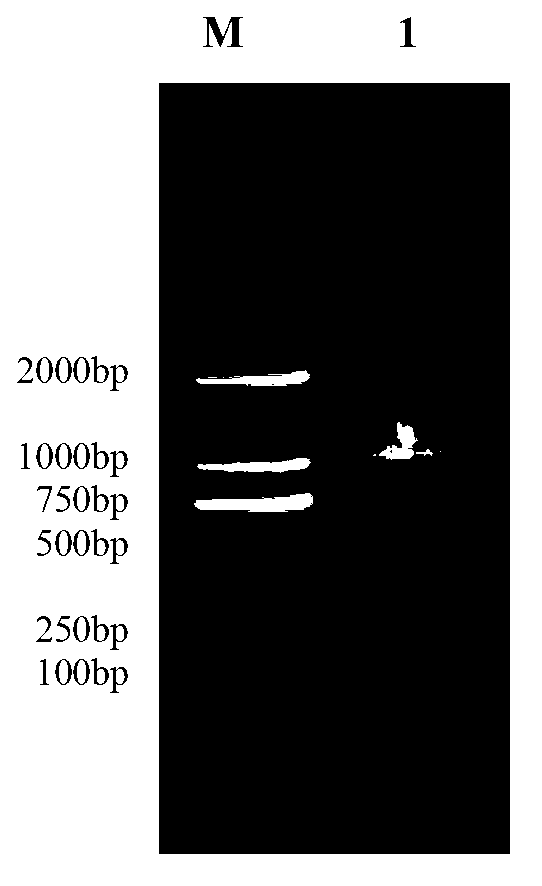
[0032] Grind 0.1 g of Litopenaeus vannamei gill tissue into powder with liquid nitrogen, add 1 mL of Trizol extract (purchased from Invitrogen, product number: 15596-026), and place it at r...
Embodiment 2
[0043] Example 2: Construction of prokaryotic recombinant expression vector pET-32a-LvMT
[0044] The recombinant vector pMD18-LvMT and the prokaryotic expression vector pET-32a (purchased from Tiangen Biochemical Technology Co., Ltd., catalog number: v1078) were double digested with BamHI restriction endonuclease and HindIII restriction endonuclease respectively to obtain the LvMT gene fragment and linear pET-32a double-digested fragments, the specific steps are: add recombinant vector pMD18-LvMT 2μl (100ng / μl), BamHI restriction enzyme 0.5μl, HindIII restriction endonuclease to 0.5mL centrifuge tube 0.5μl, 10×K buffer 2μl, using ddH 2 Make up O to 20 μl and react at 37°C for 8h. Use 1.2% agarose gel electrophoresis for detection, use an agarose gel recovery kit to recover LvMT gene and prokaryotic expression vector pET-32a, and connect the recovered fragments. The specific steps are: 3 μl (about 300ng) of LvMT gene fragments, prokaryotic Expression vector pET-32a1μl (100ng...
Embodiment 3
[0048] Example 3: Induced expression of prokaryotic recombinant expression vector pET-32a-LvMT
[0049] Take 500 μl of the Escherichia coli BL21 bacterial liquid transformed with the prokaryotic recombinant expression vector pET-32a-LvMT and the Escherichia coli BL21 bacterial liquid transformed with the empty prokaryotic expression vector pET-32a, respectively, and place them in 30 mL of LB liquid medium, shake at 37°C and 180rpm Bed cultured for 2 hours, after the culture, take out 1ml respectively, measure the OD600 value of the bacterial solution, and when the OD600 reaches 0.6, take out 2 tubes (2ml / tube) of the bacterial solution respectively, as the sample without IPTG induction and the negative control without IPTG induction , and then add IPTG with a final concentration of 0.8mM to the remaining two bacterial solutions for induction, culture on a shaker at 30°C and 180rpm for 2.5h, and take out 2 tubes (2ml / tube) of bacterial solutions as samples induced by IPTG and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com