Novel synthetic method of o-ethoxyl benzamidine hydrochloride
A technology of o-ethoxybenzamidine hydrochloride and ethoxybenzamidine hydrochloride, applied in the field of synthesis of o-ethoxybenzamidine hydrochloride, can solve the problem of unfavorable small-scale enterprises to implement and implement Problems such as high difficulty and large equipment investment have achieved the effect of low requirements for production equipment and environmental conditions, simple process and high output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
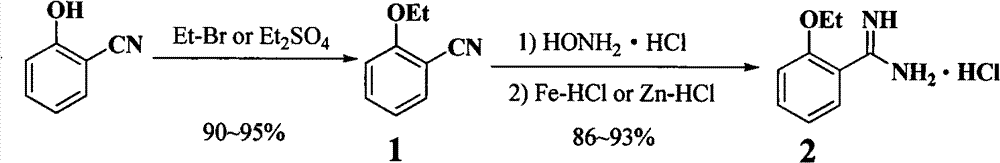
[0016] Step A: Add o-hydroxybenzonitrile (25g, 210mmol), potassium carbonate (87g, 630mmol) and ethyl bromide (34.3g, 314.8mmol) into acetone (300mL), stir, and reflux for 4 hours. After cooling and filtering, the filtrate was distilled under reduced pressure to obtain o-ethoxybenzonitrile as a colorless transparent liquid with a yield of 94%.
[0017] Step B: Add o-ethoxybenzonitrile (30g, 200mmol), hydroxylamine hydrochloride (21g, 300mmol), and potassium carbonate (41g, 300mmol) into ethanol (60mL), heat to reflux for 1.5 hours, and distill off the solvent under reduced pressure , add concentrated hydrochloric acid (30%, 200mL), slowly add zinc powder (20g) in batches under stirring, stir at room temperature for 1 hour, filter to remove unreacted zinc powder, and distill off the solvent under reduced pressure to obtain a sticky gel. Add diethyl ether (20 mL) to the liquid, filter, and collect white crystals that are o-ethoxybenzamidine hydrochloride, with a yield of 86%.
Embodiment 2
[0019] Step A: Add o-hydroxybenzonitrile (25g, 210mmol), sodium carbonate (67g, 630mmol) and ethyl bromide (34.3g, 314.8mmol) into acetone (300mL), stir and reflux for 3 hours. After cooling and filtering, the filtrate was distilled under reduced pressure to obtain o-ethoxybenzonitrile as a colorless transparent liquid with a yield of 90%.
[0020] Step B: Add o-ethoxybenzonitrile (15g, 100mmol), hydroxylamine hydrochloride (11g, 150mmol), and potassium carbonate (21g, 150mmol) into ethanol (40mL), heat to reflux for 0.5 hours, and distill off the solvent under reduced pressure , the residue was dissolved in concentrated hydrochloric acid (30%, 100mL), slowly added iron powder (10g) in batches under stirring, stirred at room temperature for 3 hours, filtered to remove the solid, distilled off most of the solvent under reduced pressure and added diethyl ether (10mL ), filtered, and the white crystals collected were o-ethoxybenzamidine hydrochloride, with a yield of 93%.
Embodiment 3
[0022] Step A: Add o-hydroxybenzonitrile (25g, 210mmol), sodium carbonate (67g, 630mmol) and diethyl sulfate (38.5g, 250mmol) into tetrahydrofuran (200mL), stir and reflux for 5 hours. After cooling and filtering, the filtrate was distilled under reduced pressure to obtain o-ethoxybenzonitrile as a colorless transparent liquid with a yield of 95%.
[0023] Step B: Add o-ethoxybenzonitrile (30g, 200mmol), hydroxylamine hydrochloride (21g, 300mmol), and potassium carbonate (41g, 300mmol) into tetrahydrofuran (60mL), heat to reflux for 1 hour, and distill off the solvent under reduced pressure , add dilute hydrochloric acid (10%, 400mL), slowly add iron powder (15g) in batches under stirring, stir at room temperature for 3 hours, filter, distill off the solvent under reduced pressure to obtain a colloidal viscous liquid, add diethyl ether (20mL ) washing, filtering, and collecting white crystals which are o-ethoxybenzamidine hydrochloride with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com