Preparation method and application of 1,4-benzodiazepine-N-nitrosamine intermediate
A technology of sodium nitrite and methylamine, applied in directions such as organic chemistry, can solve problems such as unfavorable large-scale production and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
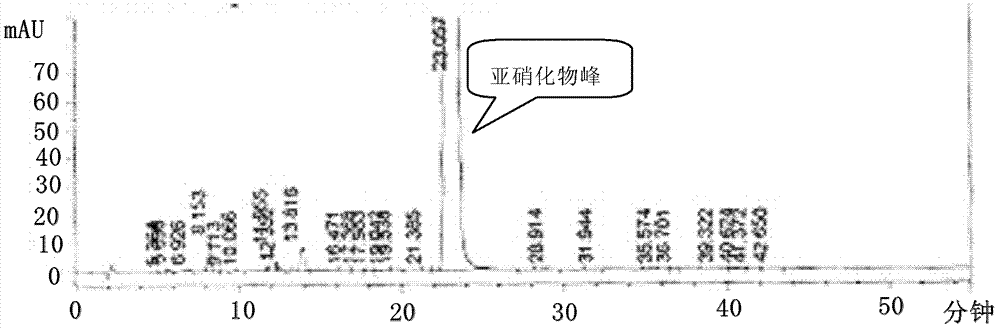
Image
Examples
preparation example Construction
[0042] Specifically, the preparation method of the compound provided by the invention with the structure shown in formula I comprises the following steps:
[0043] (a) Mix the compound shown in formula IV or formula V with an organic solvent until completely dissolved, and feed methylamine gas at 0-250°C (preferably 30-150°C) to saturation;
[0044] (b) Keep the temperature at which methylamine gas is introduced in step (a), add a mixed solution of titanium tetrachloride and the same solvent as step (a), then heat up to 0-250 ° C, and stir for 20 minutes to 20 minutes Hour (preferably reaction temperature is 1 to 10 hours), obtains reaction solution 1;
[0045] (c) mixing reaction solution 1 and sodium nitrite in the presence of organic acid or inorganic acid to obtain reaction solution 2; and
[0046] (d) Mix the reaction solution 2 and water with the same solvent as step (a), extract the aqueous layer 1-4 times with the same solvent as step (a), and obtain the compound show...
Embodiment 1
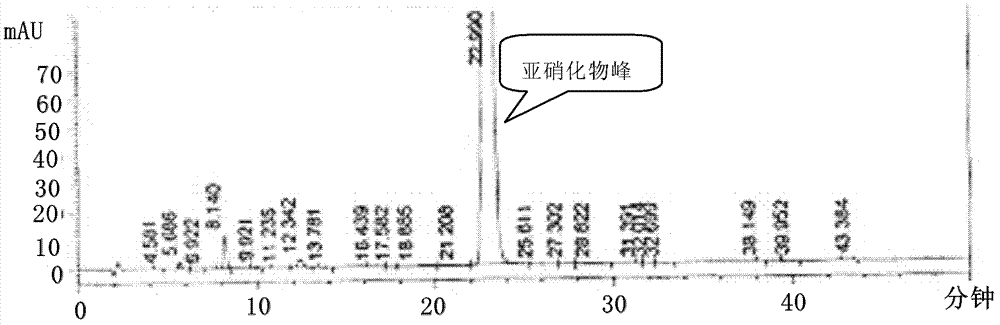
[0080] Preparation of 1,4-benzodiazepine-N-nitrosamine intermediate (II)
[0081] 1.1 Methylamination reaction
[0082] Drop into 40.5g (0.14mol) 7-chloro-5-(2-fluorophenyl)-3H-1,4-benzodiazepine-2 ketone (IV), 270ml toluene and 60mlDMF in 1L reaction bottle, room temperature Stir until completely dissolved. The temperature of the ice-salt bath was lowered to below 5°C, and methylamine gas was slowly introduced to saturation. After completion of the process, slowly add a mixture of 23ml (0.21mol) of titanium tetrachloride and 40ml of toluene dropwise while keeping the temperature below 5°C, and the temperature during the dropping process does not exceed 10°C. Then the temperature was raised to 75°C and the reaction was stirred. Timing about 3h, the reaction is complete. The reaction solution was lowered to room temperature and suction filtered, the filter cake was washed with 60ml of toluene and 30ml of DMF respectively, and the combined filtrate and washings were transfer...
Embodiment 2
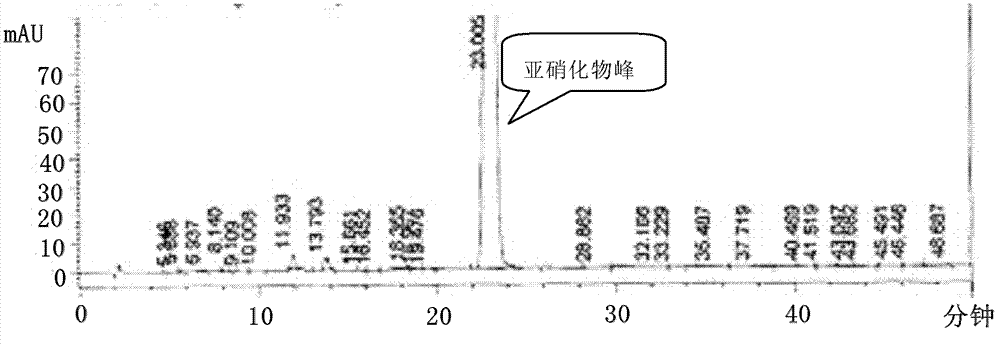
[0086] Preparation of 1,4-benzodiazepine-N-nitrosamine intermediate (III)
[0087] 2.1 Methylamination reaction
[0088] Add 37.8g (0.14mol) 7-chloro-5-(2-phenyl)-3H-1,4-benzodiazepine-2-ketone (V), 270ml toluene and 60ml tetrahydrofuran into the 1L reaction flask, room temperature Stir until completely dissolved. Cool the ice-salt bath to an internal temperature below 10°C, and slowly inject methylamine gas to saturation. After completion of the process, slowly add a mixture of 23ml (0.21mol) of titanium tetrachloride and 40ml of toluene dropwise while keeping the temperature below 10°C, and the temperature during the dropping process does not exceed 15°C. Then the temperature was raised to 75°C and the reaction was stirred. Timing about 3h, the reaction is complete. The reaction solution was lowered to room temperature and suction-filtered, the filter cake was washed with 60ml of toluene respectively, and the combined filtrate and washings were transferred to a 1L reacti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com