A new method for preparing fosfomycin levofos dexamine salt
A technology of levophosphorylamine salt and fosfomycin, which is applied in the field of preparation of fosfomycin levophosphorylamine salt, which can solve the problems of increased cost, fewer reaction steps, and low resolution rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
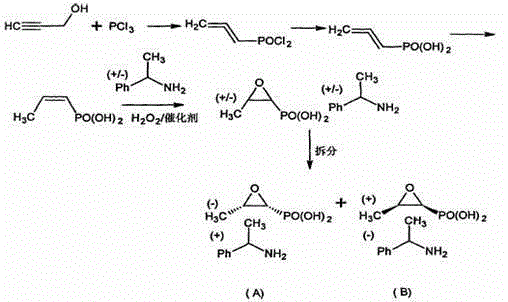
[0019] Set forth the present invention with example below:
[0020] 1. Synthesis of compound 1:
[0021] Take 1.2g (10mmol) cis-acrylophosphoric acid and absolute ethanol (5ml) into a 50ml three-necked bottle, stir to dissolve completely. Add dropwise by 0.5gNa 2 WO 4 Dissolve 0.13g EDTA-2Na in 5ml of water, then add 1.7g (15mmol) of 30% hydrogen peroxide, heat to 40 degrees to completely dissolve, and react for 1h. The reaction was monitored by high performance liquid chromatography. The insoluble matter was filtered off by cooling, and the ethanol was distilled off, then ethyl acetate was added to extract the generated DS fosfomycin, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a colorless oil (1.3 g, yield 94%).
[0022] 2. Dynamic split
[0023] Add 8 milliliters of deionized water and 2 milliliters of dimethyl sulfoxide into the reaction flask, start stirring, put 1.3 g of the product of the previous step fosfomycin into it, raise the tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com