Bi-fluoranthene-containing organic semiconductor material, its preparation method and application
An organic semiconductor, double fluoranthene technology, applied in semiconductor/solid-state device manufacturing, semiconductor devices, organic chemistry, etc., can solve the problems of poor solubility, poor film formation, and low yield, and achieve simple operation, Superior performance and easy processing results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of the above-mentioned bisfluoranthene-containing organic semiconductor material comprises the following steps:
[0032] S1, the structural formula is The 2,7-bistrimethylsilylacetylene-9,9-diR base fluorene, the structural formula is Trimethylsilylacetylene, catalyst and first solvent (such as, triethylamine, namely NEt 3 ) into the reactor without error, after heating and reflux reaction in an oil bath for 48h, the structural formula is generated as 2,7-ditrimethylsilylacetylene-9,9-dialkylfluorene; wherein, the reactor is an oxygen-free environment (that is, an inert atmosphere composed of at least one gas of nitrogen and argon), 2,7- The molar ratio of ditrimethylsilylacetylene-9,9-diR base fluorene to trimethylsilylacetylene is 1:3; the reaction formula is as follows:
[0033]
[0034] S2. Dissolve 2,7-ditrimethylsilylacetylene-9,9-dialkylfluorene in a mixed solvent containing tetrahydrofuran (THF) and methanol (MeOH), and then dropw...
Embodiment 1
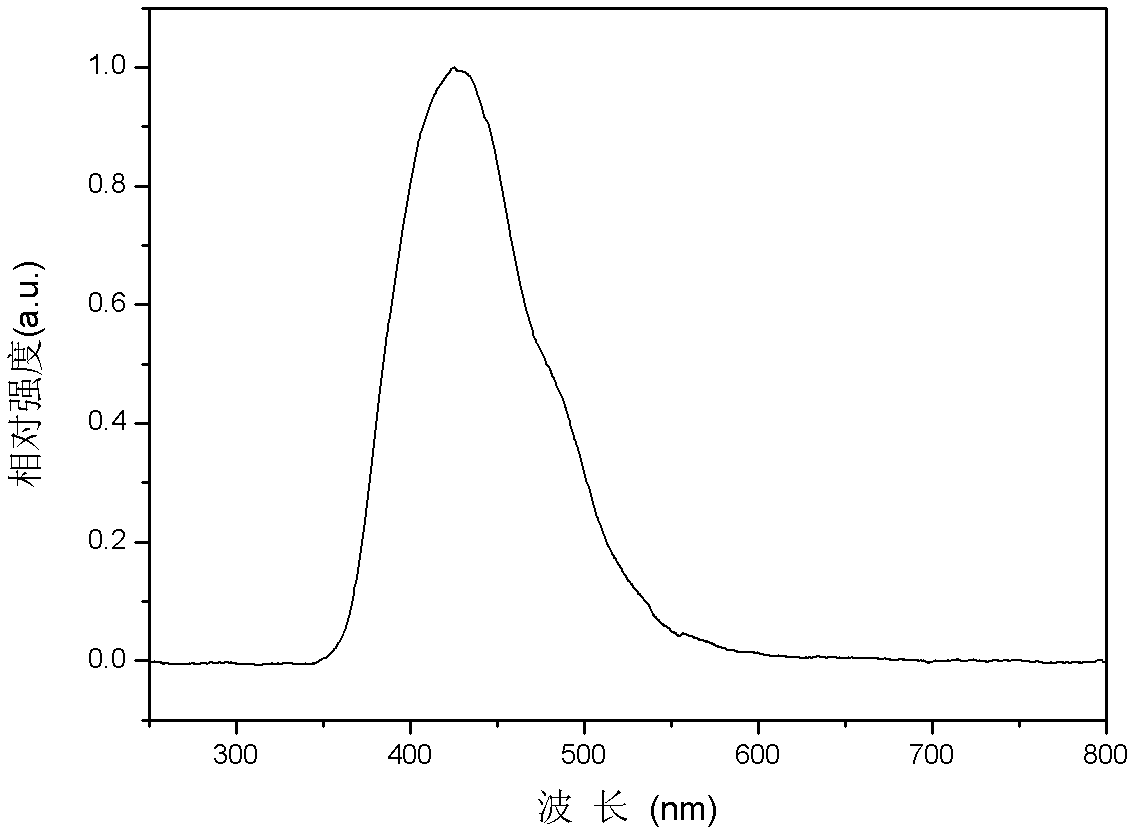
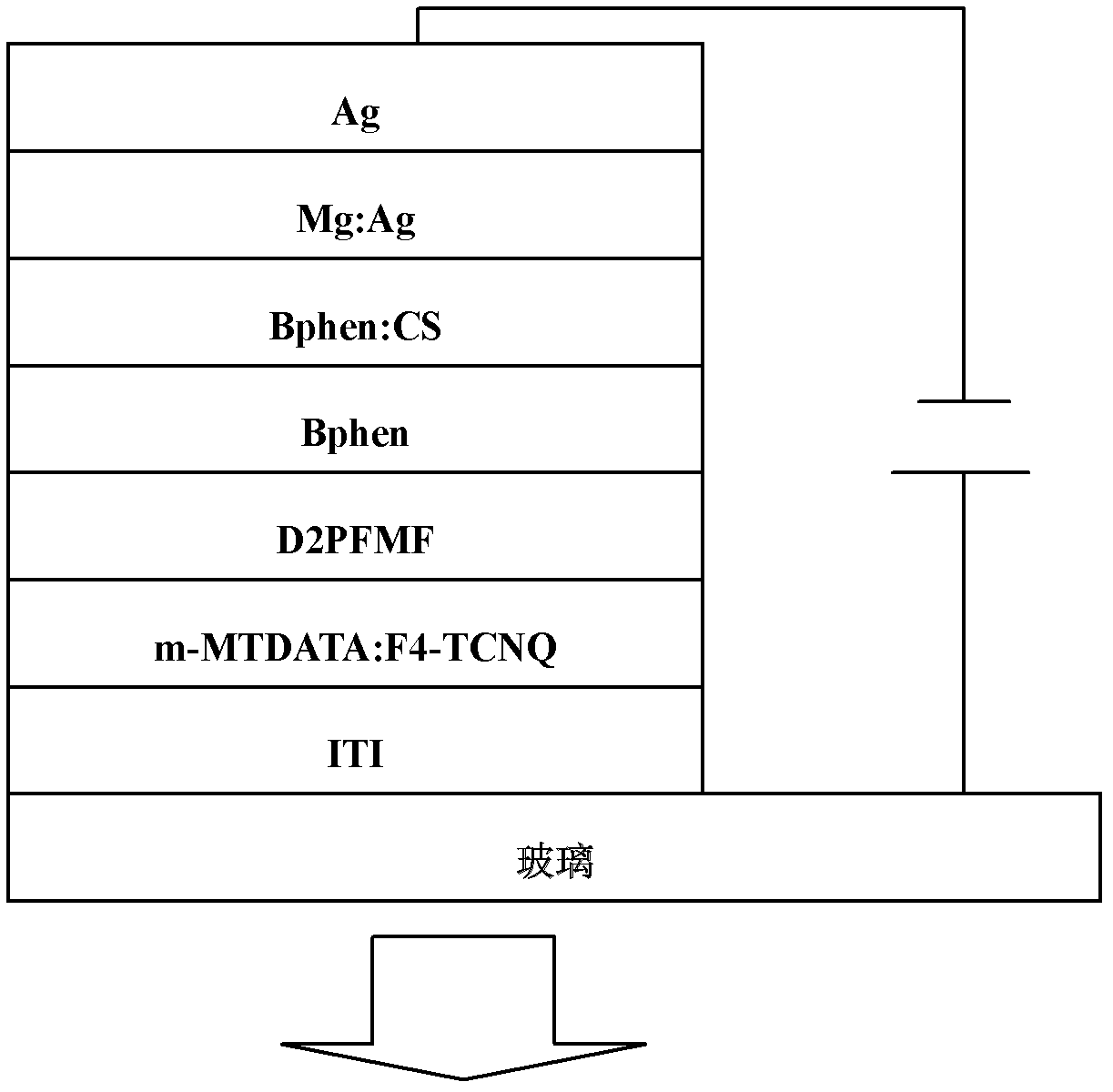
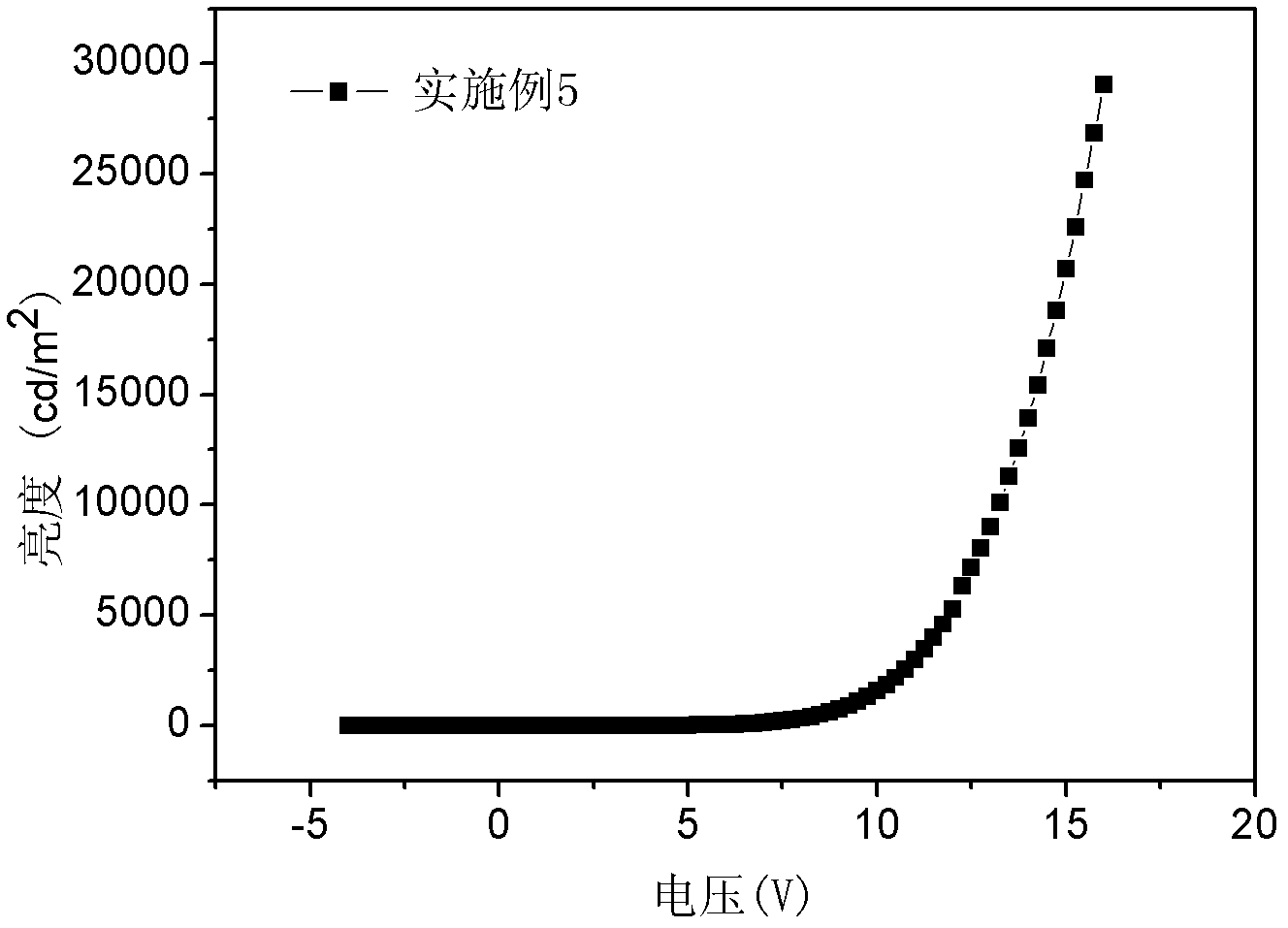
[0050] This example discloses a fluorene-containing bisfluoranthene organic compound with the following structure: 2,7-bis(7,10-diphenylfluoranthene)-9,9-dimethylfluorene (D2PFMF)
[0051]
[0052] Step 1: Preparation of Compound A: 2,7-Ditrimethylsilylacetylene-9,9-Dimethylfluorene
[0053]
[0054] In a three-necked flask with nitrogen, add the catalyst Pd(PPh 3 ) 2 Cl 2 (630mg, 0.9mmol), CuI (45mg, 0.45mmol), 2,7-dibromo-9,9-dimethylfluorene (5.28g, 15mmol), trimethylsilylacetylene (4.42g, 45mmol), and 60mL triethylamine was used as a solvent, vacuumed three times with nitrogen gas, heated to reflux in an oil bath for 48h, washed with ether and filtered, the solvent was spin-off, and n-hexane was used as an eluent for silica gel column chromatography to obtain a light yellow solid (4.5, yield 78 %). MS: m / z386. 1 H NMR (400MHz, CDCl 3 ) δ (ppm): 7.81 (d, 2H), 7.70 (d, 2H), 7.49 (t, 2H), 1.97 (s, 6H), 0.19 (s, 18H).
[0055] Step 2: Preparation of 2,7-bis(7,10-d...
Embodiment 2
[0061] This example discloses a fluorene-containing bisfluoranthene organic compound with the following structure: 2,7-bis(7,10-diphenylfluoranthene)-9,9-diethylfluorene (D2PFEF)
[0062]
[0063] Step 1: Preparation of Compound A: 2,7-Ditrimethylsilylacetylene-9,9-Diethylfluorene
[0064]
[0065] Into a three-necked flask with argon gas, add catalyst Pd(PPh 3 ) 2 Cl 2 (630mg, 0.9mmol), CuI (45mg, 0.45mmol), 2,7-dibromo-9,9-diethylfluorene (5.70g, 15mmol), trimethylsilylacetylene (4.42g, 45mmol), and 60mL of triethylamine was used as a solvent, evacuated and ventilated with argon for three times, heated and refluxed in an oil bath for 48h, washed with ether and filtered, the solvent was spin-off, and using n-hexane as an eluent for silica gel column chromatography to obtain a light yellow solid (4.97, the yield 80%). MS: m / z 414. 1 H NMR (400MHz, CDCl 3 ) δ (ppm): 7.81 (d, 2H), 7.70 (d, 2H), 7.49 (t, 2H), 1.97 (q, 4H), 1.41 (t, 6H), 0.19 (s, 18H).
[0066] Step 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com