Synthetic method of anti-tumor medicament lonidamine
A synthesis method and anti-tumor technology, applied in the direction of organic chemistry, etc., can solve the problems of high production cost of lonidamine, achieve the effects of shortening the synthesis time, increasing the reaction yield, and cheap price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
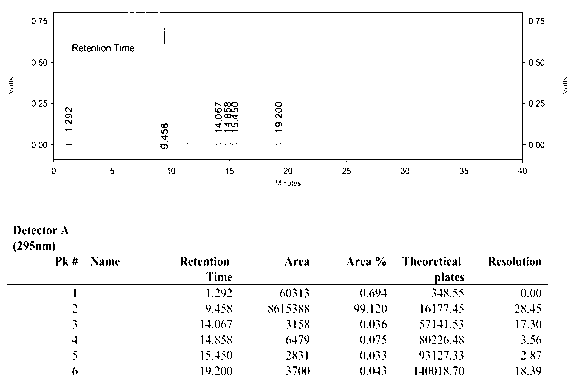
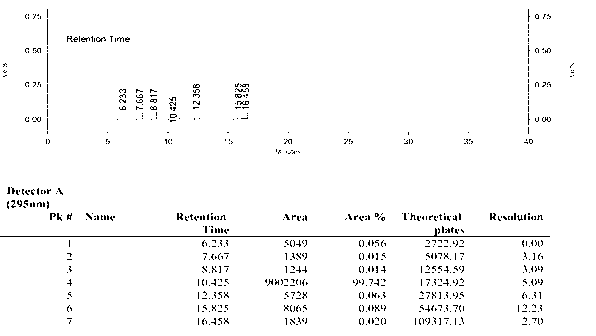
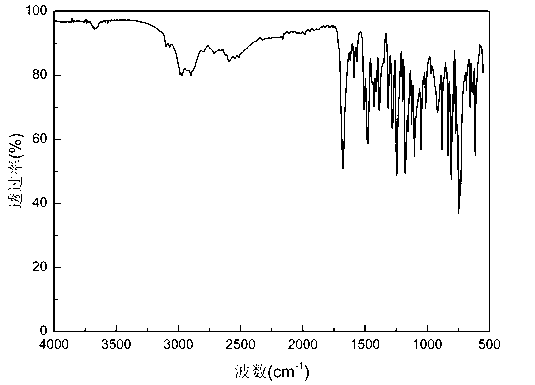
Image
Examples
Embodiment 1
[0045]Synthesis of 2,4-dichlorobenzyl bromide:
[0046] (1) 10.0g (0.06mol) 2,4-dichlorotoluene, 9.95g (0.072mol) NBS, 1.0g
[0047] Azodiisobutyronitrile and 100g dichloroethane were put into a 250ml four-neck bottle, and the temperature was raised to 80°C under stirring, and the reaction was stopped after 8 hours of heat preservation, and filtered; the mother liquor was washed with water, dilute sodium hydroxide solution, and saturated sodium sulfite solution respectively. The organic phase was separated several times, and the solvent dichloroethane was distilled off to obtain 14.2 g of light yellow 2,4-dichlorobenzyl bromide liquid. Yield: 80.1%.
[0048] (2) 10.0g (0.06mol) 2,4-dichlorotoluene, 13.26g (0.072mol) NBS, 1.0g
[0049] Azobisisobutyronitrile and 100g of dichloroethane were thrown into a 250ml four-necked bottle, and the temperature was raised to 80°C under stirring, and the reaction was stopped after 6 hours of heat preservation, and filtered; the mother liqu...
Embodiment 2
[0051] Synthesis of N-acetylphenylhydrazine:
[0052] (1) Add 98.0g (1.55mol) of glacial acetic acid and 100.0g of water into a 250ml three-necked flask, and add benzene under stirring
[0053] Hydrazine 40.0g (0.37mol), heated to reflux, kept warm for 4h, cooled to room temperature, a large amount of yellow solid precipitated, filtered, washed with water, dried to obtain 80.0g N-acetylphenylhydrazine, yield: 82.0%, melting point: 127℃~ 129°C.
[0054] (2) Add 58.5g (0.92mol) of glacial acetic acid and 100.0g of water into a 250ml three-neck flask, add benzene under stirring
[0055] Hydrazine 40.0g (0.37mol), heated to reflux, kept warm for 4h, cooled to room temperature, a large amount of yellow solid precipitated, filtered, washed with water, dried to obtain 58.5g of N-acetylphenylhydrazine, yield: 60.0%, melting point: 127℃~129 ℃.
Embodiment 3
[0057] Synthesis of N-acetylaminoxime acetanilide:
[0058] (1) Add 5.0g (0.033mol) acetylphenylhydrazine, 40g (0.282mol) anhydrous sodium sulfate, 7.5g (0.108mol) hydroxylamine hydrochloride, 6g (0.100mol) glacial acetic acid and 125.0g Add water, stir and raise the temperature to 90°C; then add 7.2g (0.044mol) chloral hydrate dissolved in 14.4g water dropwise into the reaction solution, the drop is completed in about 5 minutes, start timing with the drop, and stop after 15 minutes of reaction For reaction, immediately pour the reaction solution into a certain amount of water, cool to room temperature, filter, wash with water, and dry to obtain 6.40 g of light yellow solid, yield: 86.9%, melting point: 133°C-135°C.
[0059] (2) Add 5.0g (0.033mol) acetylphenylhydrazine, 35g (0.282mol) anhydrous sodium sulfate, 7.5g (0.108mol) hydroxylamine hydrochloride, 6g (0.100mol) glacial acetic acid and 125.0g Add water, stir and raise the temperature to 90°C; then add 7.2g (0.044mol) c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com