Thiophene pyrroledione based co-polymer material, and preparation method and application thereof
A pyrrole diketo-based and copolymer technology is applied in the field of solar cell materials, which can solve the problems of low conversion efficiency of inorganic solar cells and the like, and achieve the effects of excellent photovoltaic performance, improved open circuit voltage and improved energy conversion efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
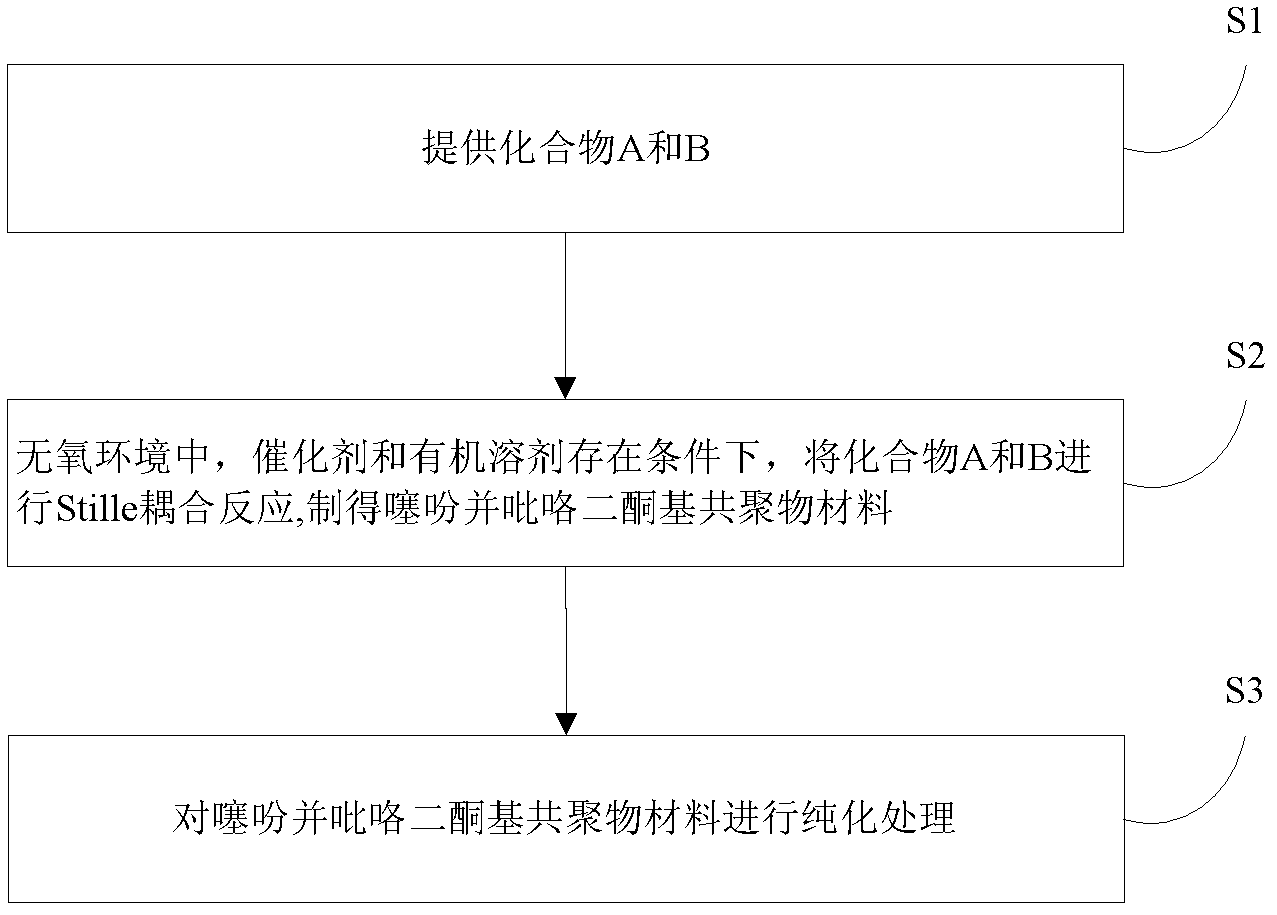
[0031] The preparation method of above-mentioned thienopyrrole diketopyl copolymer material, such as figure 1 shown, including the following steps:
[0032] S1, respectively provide compound A and compound B represented by the following structural formula,
[0033] 2,6-Ditrimethyltin-4,8-dialkoxybenzodithiophene
[0034] N-Alkyl-thieno[3,4-c]pyrrole-4,6-dione
[0035] Among them, in compound A, R 1 for C 1 ~C 20 The alkyl group; in compound B, R 2 for C 1 ~C 20 the alkyl group;
[0036] S2. In an oxygen-free environment (such as an oxygen-free environment composed of nitrogen, argon, or a mixture of nitrogen and argon), the compound A and compound B are added in a molar ratio of 1:1 into the catalyst containing In the organic solvent, after fully dissolving, carry out Stille coupling reaction at 70~130 ℃ for 6~60h, after cooling down to stop the reaction, a mixed solution is obtained, and the mixed solution contains the product, that is, the thiophene having the f...
Embodiment 1
[0050] The thienopyrrole diketopyl copolymer material of this embodiment, that is, poly{4,8-di(octyloxy)benzodithiophene-co-N-octyl-thiophene[3,4-c]pyrrole- 4,6-diketone} (wherein, R 1 is n-octyl, R 2 Be n-octyl, n is 60), its structural formula is as follows:
[0051]
[0052] The preparation steps of above-mentioned polymer are as follows:
[0053] The reaction formula is as follows:
[0054]
[0055] 2,6-Ditrimethyltin-4,8-bis(n-octyloxy)benzodithiophene (232mg, 0.3mmol), N-n-octyl-2,5-dibromo-thiophene[3, 4-c] pyrrole-4,6-dione (126mg, 0.3mmol), tridibenzylideneacetone dipalladium (13.75mg, 0.015mmol) and tri-tert-butylphosphine (24.2mg, 0.12mmol) were added to the Dissolve in a flask with 12mL of toluene to form a solution, fully ventilate nitrogen into the flask for about 30 minutes, stir at 95°C, and conduct Stille coupling reaction for 40 hours. After cooling down, stop the polymerization reaction to obtain a mixed solution.
[0056] Add 40mL of methanol to th...
Embodiment 2
[0060] The thienopyrrole diketopecopolymer material of this embodiment, that is, poly{4,8-di(methoxy)benzodithiophene-co-N-n-eicosyl-thiophene[3,4-c] And pyrrole-4,6-dione} (wherein, R 1 is methyl, R 2 Be n-eicosyl, n is 40), its structural formula is as follows:
[0061]
[0062] The preparation steps of above-mentioned polymer are as follows:
[0063] The reaction formula is as follows:
[0064]
[0065] 2,6-Ditrimethyltin-4,8-bis(methoxy)benzodithiophene (115mg, 0.2mmol) and N-n-eicosyl-2,5-dibromo-thiophene[3 ,4-c] pyrrole-4,6-dione (118mg, 0.2mmol) was added into a 15ml N,N-dimethylformamide flask, dissolved into a solution, and the flask was evacuated to remove oxygen and filled with argon Then add bistriphenylphosphinepalladium dichloride (5.6mg, 0.008mmol), stir at 120°C, conduct Stille coupling reaction for 12h, stop the polymerization reaction after cooling down, and obtain a mixed solution.
[0066] Add 50mL of methanol into the flask, carry out precipita...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com