Application of complex of parietic acid or parietic acid compounds and arginine in preparation of medicines for treating vascular complications of diabetes
A technology for diabetic blood vessels and rhein is applied in the application field of rhein or a compound of rhein and arginine in the preparation of medicines for treating diabetic vascular complications, and can solve problems such as male sexual dysfunction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
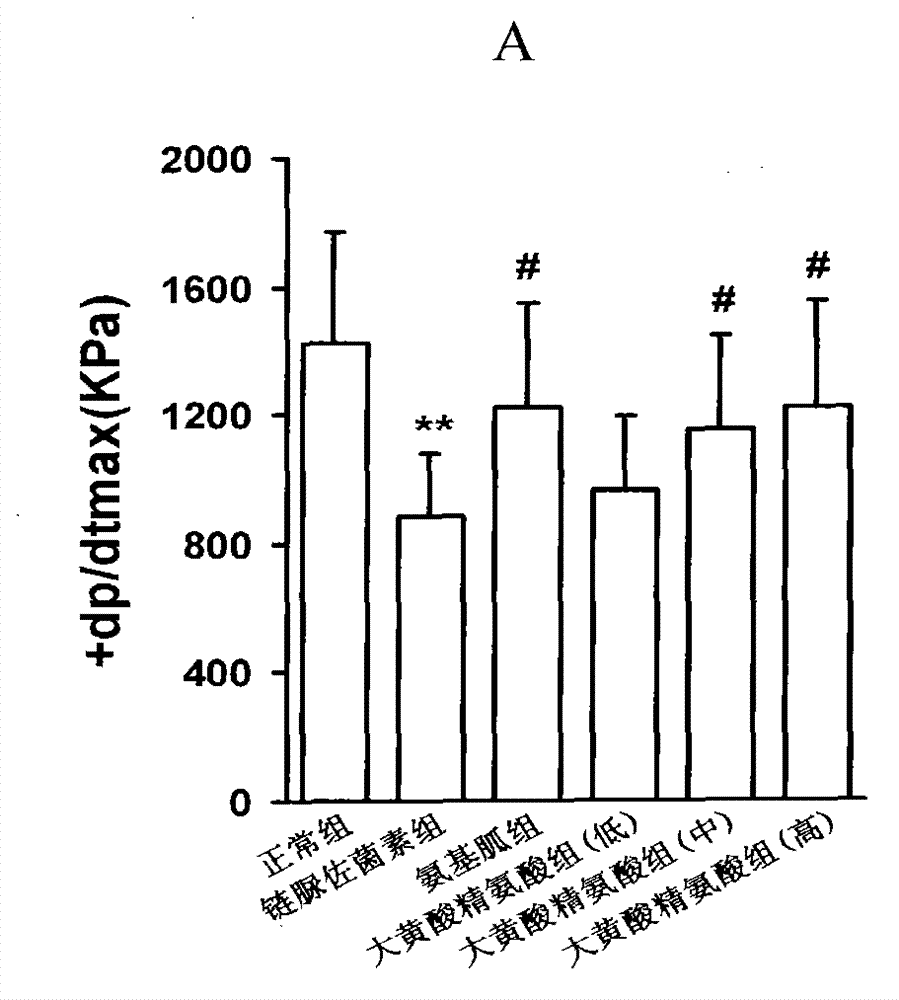
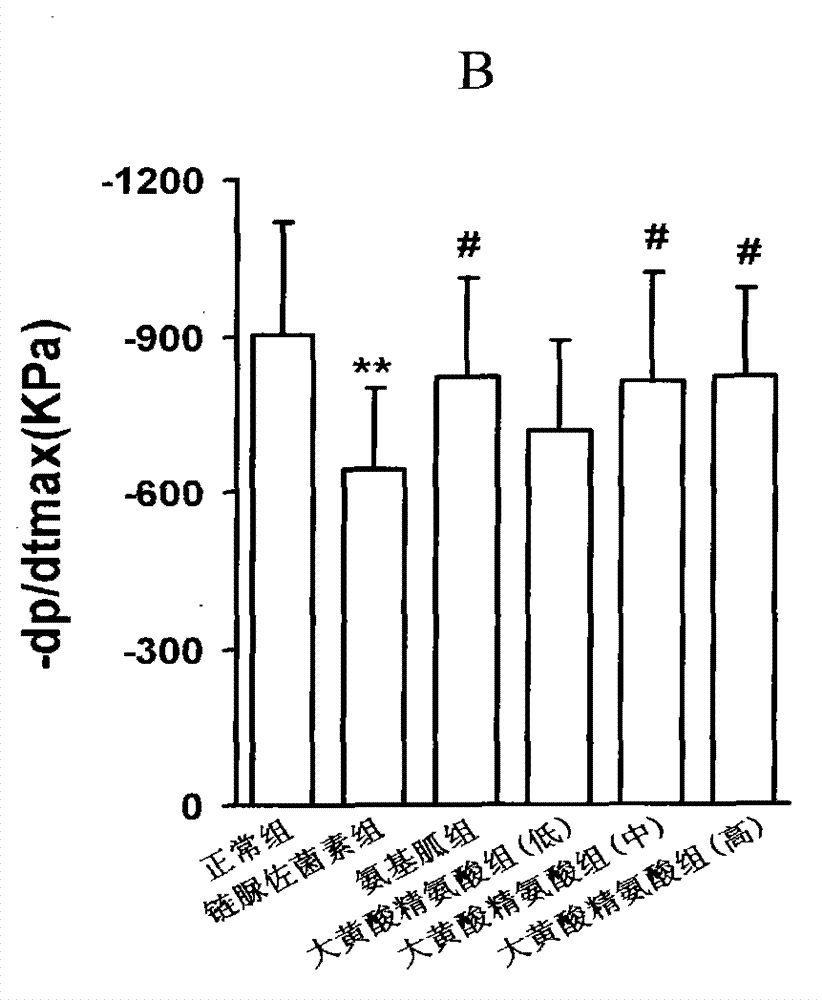
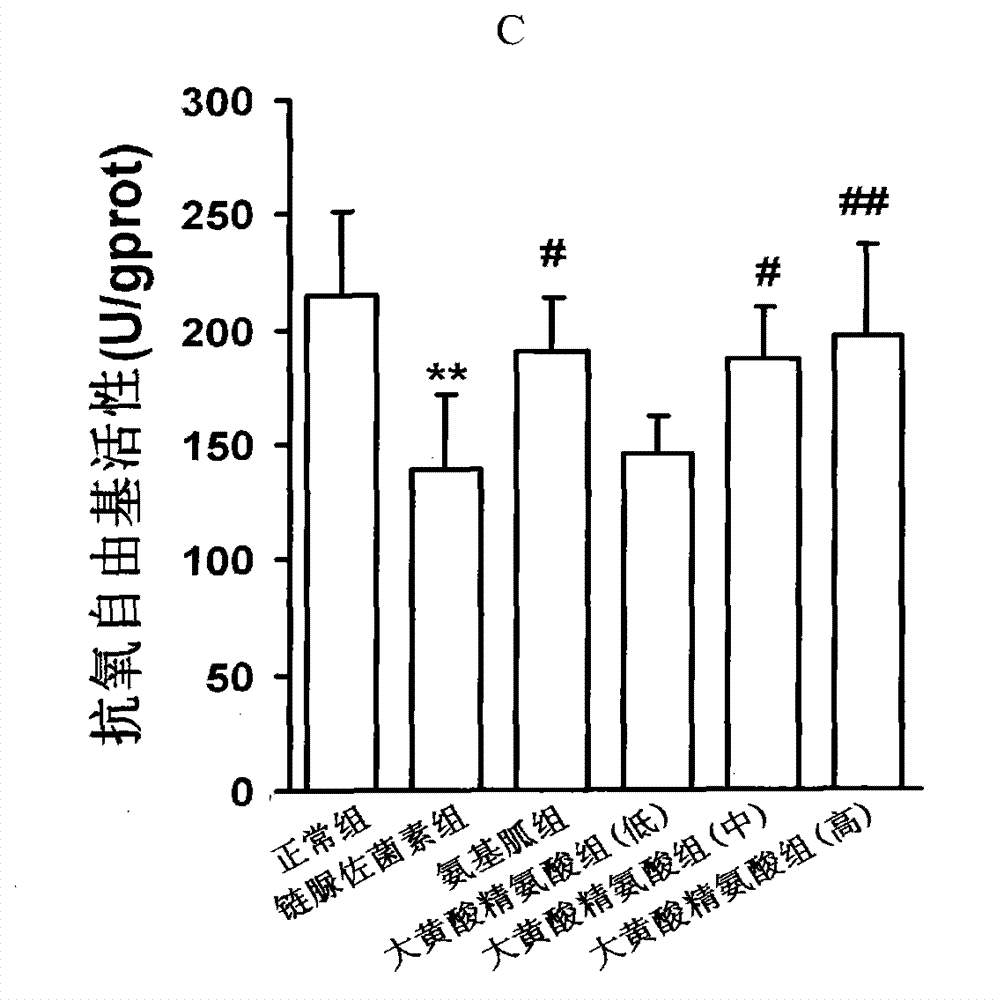
[0094] Example 1, the compound of rhein and arginine (macroarginic acid) treats diabetic cardiomyopathy.
[0095] Diabetes was induced in rats by intraperitoneal injection of streptozotocin 65mg / kg. Blood sugar was as high as 25mM and maintained for 8 weeks. After 4 weeks, diabetic complications had appeared. The untreated group continued to develop. The treatment group was administered in the last 4 weeks (mg / kg, intragastric administration): positive drug aminoguanidine 100, and 3 doses of sarginic acid: 50 mg / kg, 100 mg / kg, 200 mg / kg. Although the untreated diabetic group did not receive medication, they controlled their food intake and were given dietary control treatments to obtain appropriate treatment.
[0096] Pharmacodynamic observation: It consists of macroscopic main pharmacodynamic indicators, expression of important bioactive molecules (mRNA, and protein) in diseased cells and other indicators.
[0097] Sarginic acid restored the above indicators to the normal...
Embodiment 2
[0099] Example 2, the treatment of diabetic macroangiopathy with sarginic acid. Basically the same as Example 1,
[0100] Diabetes was induced in rats by intraperitoneal injection of streptozotocin 65mg / kg. Blood sugar was as high as 25mM and maintained for 8 weeks. After 4 weeks, diabetic complications had appeared. The untreated group continued to develop. The treatment group was administered in the last 4 weeks (mg / kg, intragastric administration): positive drug aminoguanidine 100, and 3 doses of sarginic acid: 50 mg / kg, 100 mg / kg, 200 mg / kg. Although the untreated diabetic group did not receive medication, they controlled their food intake and were given dietary control treatments to obtain appropriate treatment.
[0101] The vasodilation function and NO bioavailability were restored after 4 weeks of treatment with sarginic acid and aminoguanidine. At the same time, the molecular biological targets abnormally expressed in the blood vessel wall are basically restored t...
Embodiment 3
[0103] Example 3, the treatment of diabetic nephropathy with macrospermic acid.
[0104] Diabetes was induced in rats by intraperitoneal injection of streptozotocin 65mg / kg. Blood sugar was as high as 25mM and maintained for 8 weeks. After 4 weeks, diabetic complications had appeared. The untreated group continued to develop. The treatment group was administered in the last 4 weeks (mg / kg, gavage): positive drug aminoguanidine 100, 3 dosage groups of spermatic acid: 50mg / kg, 100mg / kg, 200mg / kg. Although the untreated group of diabetes did not take drugs, they controlled their food intake and were given diet control treatment to obtain appropriate treatment.
[0105]The trace protein in 24h urine of rats with diabetic nephropathy increased significantly, and the creatinine and non-protein nitrogen in blood increased, suggesting: diabetic nephropathy. At the same time, the molecular biological indicators: the gene and protein expression of PPARa in the kidney, the expression...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com