Method for preparing side-chain amylose with different carbamates and chiral stationary phase
A carbamate and amylose technology, applied in chemical instruments and methods, and other chemical processes, can solve problems such as poor chiral recognition ability, and achieve the effects of easy control, high yield, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
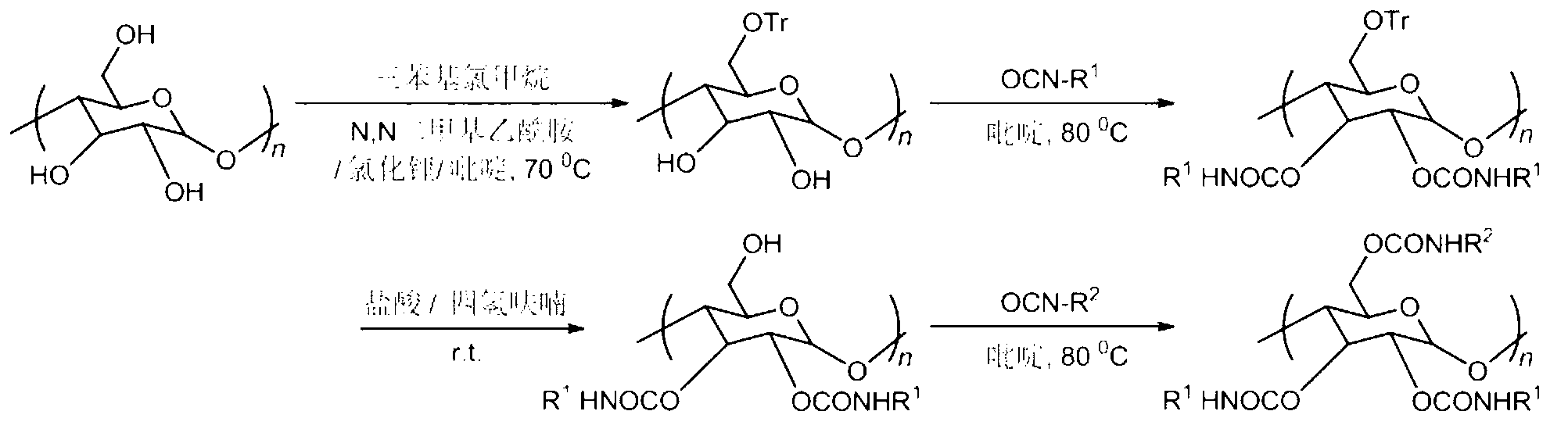
[0021] 1. Take 0.2g amylose and dry it in vacuum at 80°C for 4h, then stir and reflux in anhydrous N,N-dimethylacetamide for 12h; add 0.4g lithium chloride after cooling to room temperature; continue stirring for 2h , reheat to 70°C, add anhydrous pyridine, add excess triphenylchloromethane after reflux for 4h, stop the reaction after continuous stirring and reflux for 24h; cool to room temperature, add methanol to settle, filter and wash, and vacuum dry at 60°C to Constant weight, yield 88%.
[0022] 2. Continue vacuum drying the above intermediate product at 80°C for 4h, then reflux in anhydrous pyridine for 3h, then add excess 3,5-dimethylphenylisocyanate, continue to reflux at 80°C for 16h, then stop the reaction , washed well with methanol and dried in vacuo.
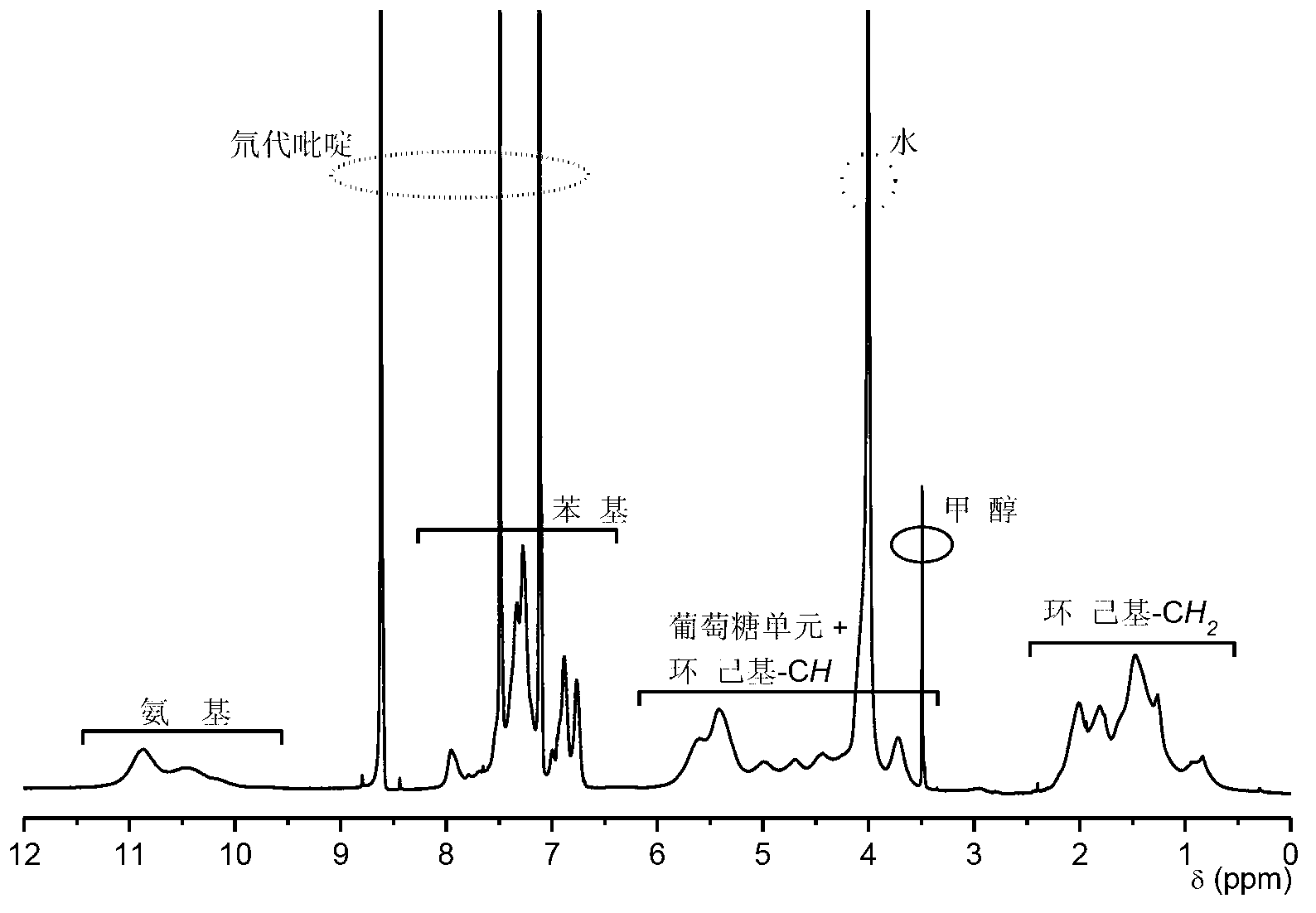
[0023] 3. The intermediate product obtained in the previous step was dissolved in a tetrahydrofuran solution containing a small amount of hydrochloric acid (1.8% by volume of tetrahydrofuran) for hydrolysis, and s...
specific Embodiment approach 2
[0027] 1. Take 0.2g amylose and dry it in vacuum at 80°C for 4h, then stir and reflux in anhydrous N,N-dimethylacetamide for 10h; add 0.4g lithium chloride after cooling to room temperature; continue stirring for 4h , reheat to 70°C, add anhydrous pyridine, add excess triphenylchloromethane after reflux for 3h, stop the reaction after continuous stirring and reflux for 24h; cool to room temperature, add methanol to settle, filter and wash, and vacuum dry at 60°C to Constant weight, yield 85%.
[0028] 2. Continue vacuum drying the above intermediate product at 80°C for 3h, then add excess 3,5-dichlorophenylisocyanate after reflux in anhydrous pyridine for 3h, stop the reaction after continuing to reflux at 80°C for 12h, Wash well with methanol and dry under vacuum.
[0029] 3. The intermediate product obtained in the previous step was dissolved in a tetrahydrofuran solution containing a small amount of hydrochloric acid (1.8% by volume of tetrahydrofuran) for hydrolysis, and ...
specific Embodiment approach 3
[0033] 1. Take 0.2g amylose and dry it in vacuum at 80°C for 4h, then stir and reflux in anhydrous N,N-dimethylacetamide for 10h; add 0.4g lithium chloride after cooling to room temperature; continue stirring for 4h , reheat to 70°C, add anhydrous pyridine, add excess triphenylchloromethane after reflux for 4h, stop the reaction after continuous stirring and reflux for 23h; cool to room temperature, add methanol to settle, filter and wash, and vacuum dry at 60°C to Constant weight, yield 90%.
[0034] 2. Continue to vacuum-dry the above-mentioned intermediate product at 80°C for 3 hours, then reflux in anhydrous pyridine for 4 hours, add excess 4-chlorophenyl isocyanate, continue to reflux at 80°C for 12 hours, and then stop the reaction. Wash and vacuum dry.
[0035] 3. The intermediate product obtained in the previous step was dissolved in a tetrahydrofuran solution containing a small amount of hydrochloric acid (1.9% by volume of tetrahydrofuran) for hydrolysis, and stirre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com