Biodegradable polyurethane with amino on side chain and preparation method and application thereof
A biodegradable, polyurethane technology, applied in the field of biomedical materials, to achieve the effects of low physiological toxicity, regular structure, high availability and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
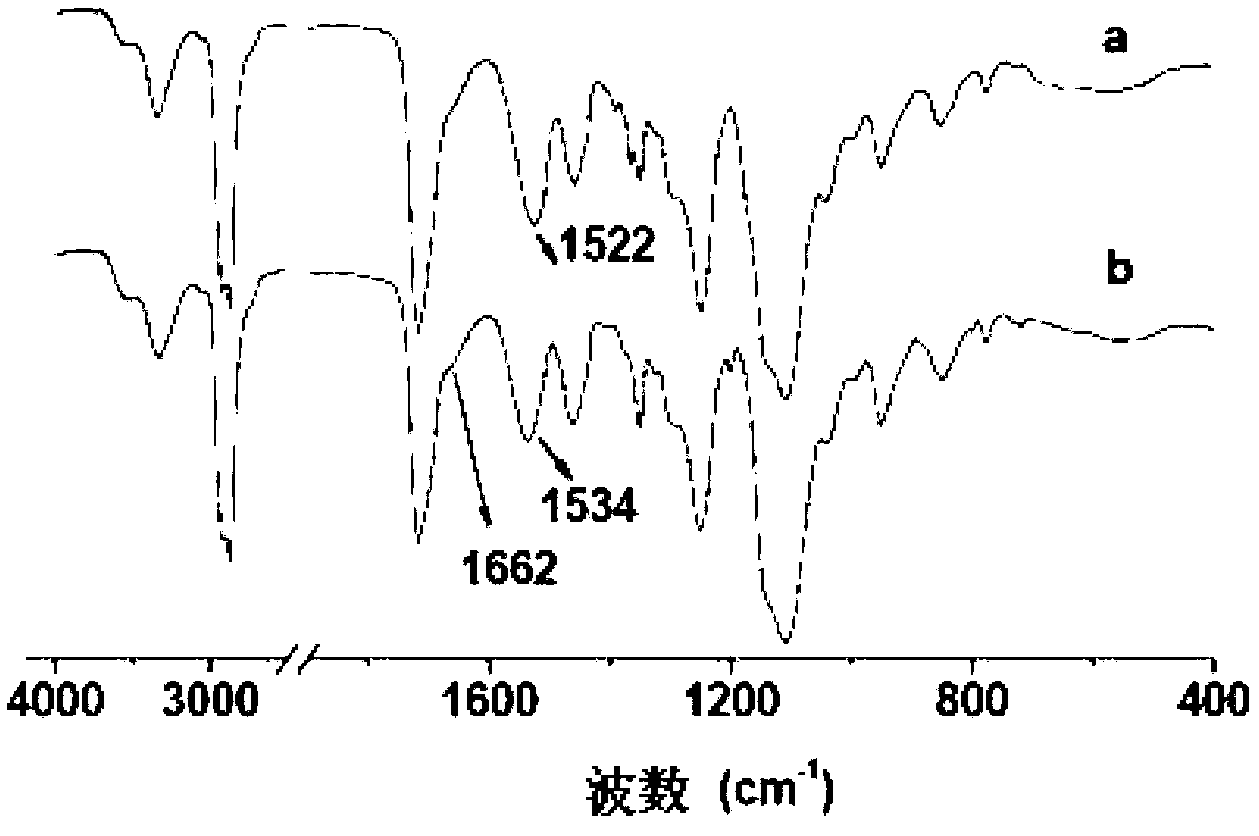
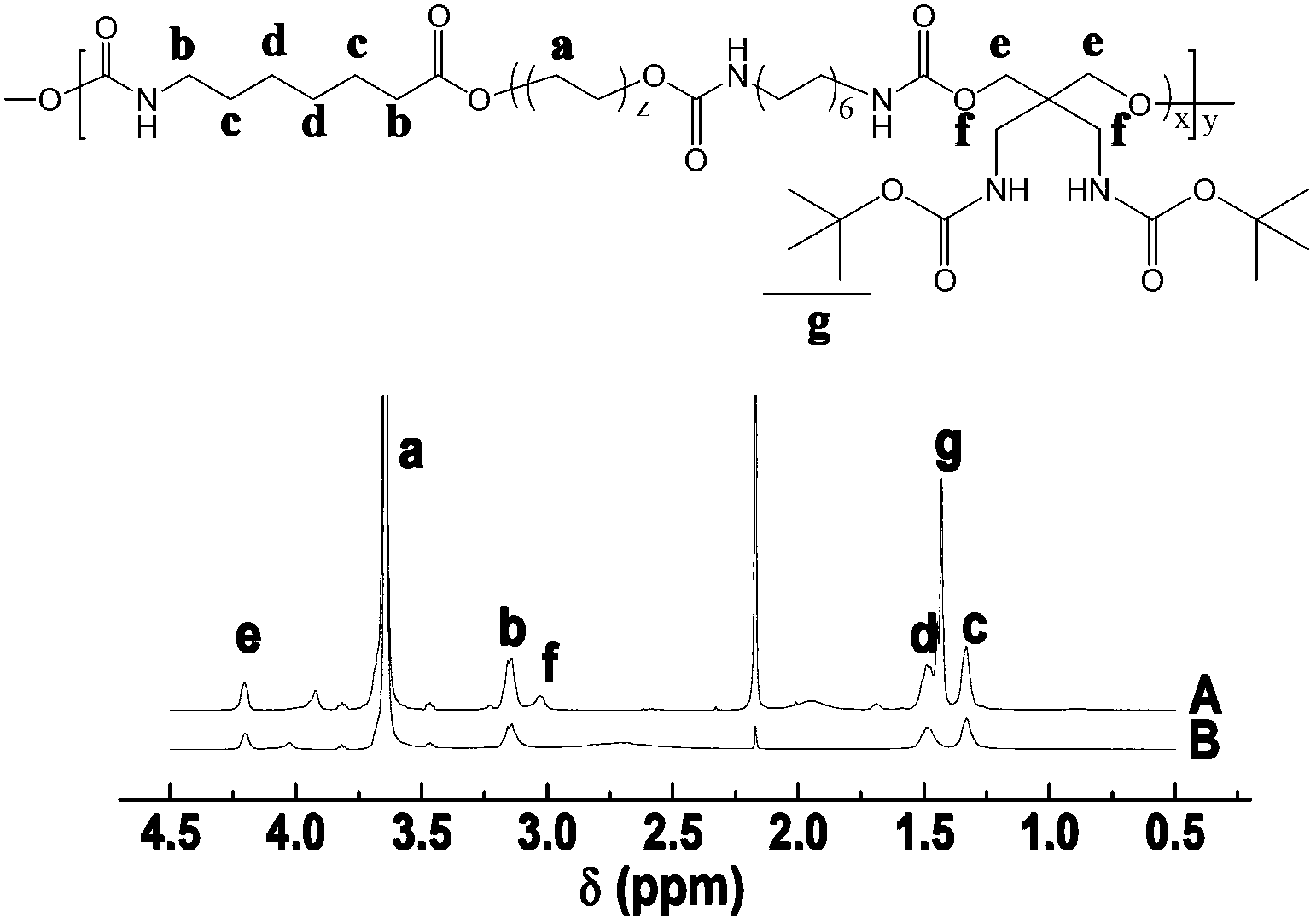
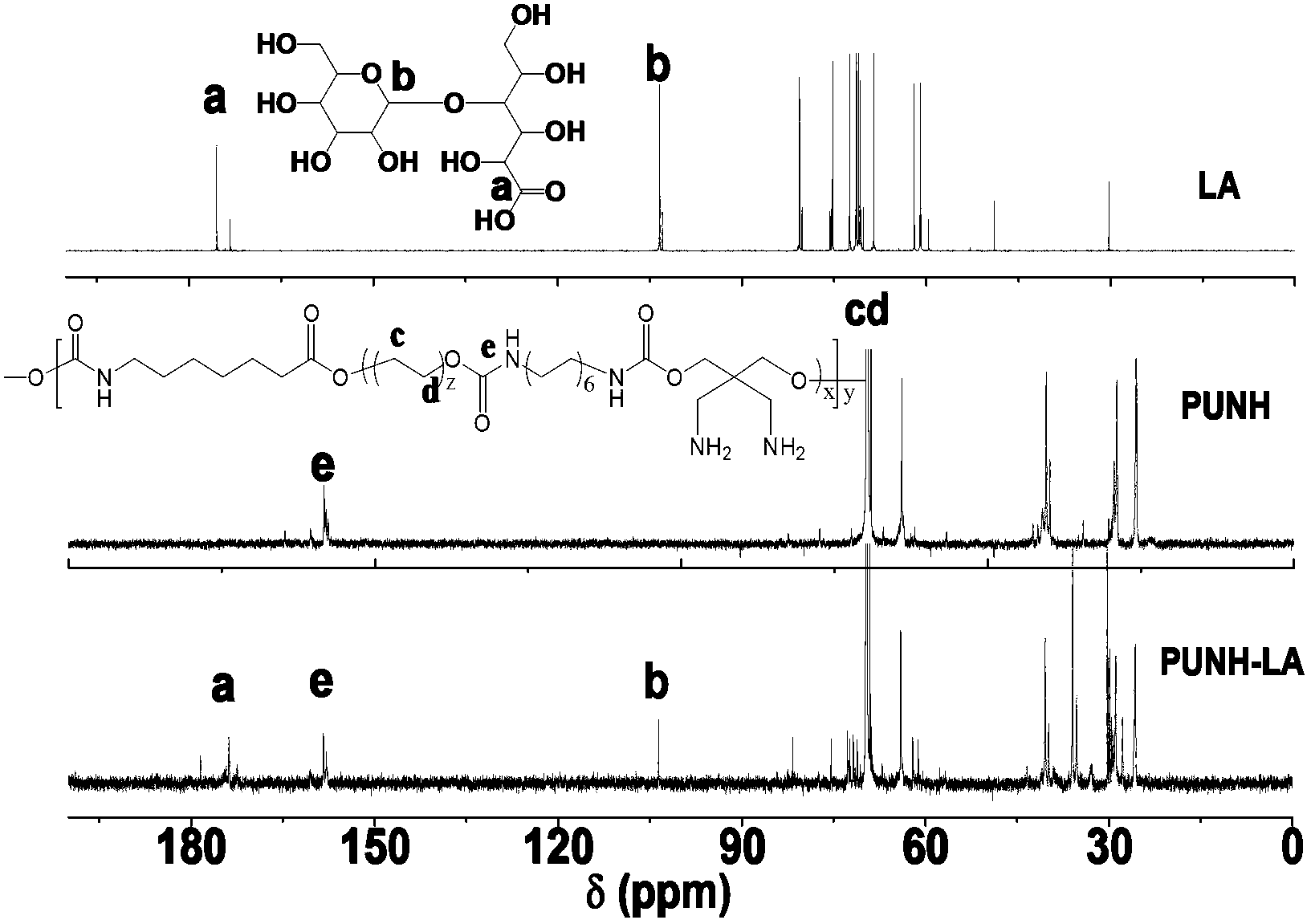
Image
Examples
Embodiment 1
[0080] Example 1: Preparation of polyester polyurethane with polylactide diol as soft segment with protected amino group in side chain
[0081] Put dry 0.01mol of polylactic acid diol (Mn=0.3kg / mol) in a dry 50ml reaction bottle, add 10ml of anhydrous toluene, wait until it is completely dissolved, add 0.015mol of lysine methyl diisocyanate and 0.15g stannous octoate, 70 ℃, reacted for 0.5 hours, then added 0.005mol chain extender 2,2-(2-chlorobenzyloxycarbonyl) diaminomethyl-1,3-propanediol to the reaction flask, reaction 1 Hours, the product was precipitated in ethanol to obtain polyester polyurethane with polylactide diol as the soft segment.
Embodiment 2
[0082] Embodiment 2: Preparation of polyester polyurethane with polycaprolactone diol as soft segment with protected amino group in side chain
[0083] Put dry 0.02mol of polycaprolactone diol (Mn=5kg / mol) in a dry 500ml reaction bottle, add 250ml of anhydrous tetrahydrofuran, wait until it is completely dissolved, add 0.04mol of isophorone diisocyanate and 0.015g dibutyltin dilaurate, 80°C, react for 2 hours, then add 0.02mol of chain extender 2,2-benzyloxycarbonyl diaminomethyl-1,3-propanediol to the reaction flask, react for 4 hours, the product Precipitate in ethanol to obtain polyester polyurethane with polycaprolactone diol as the soft segment.
Embodiment 3
[0084] Example 3: Preparation of Polyester Polyurethane with Polycarbonate Diol as Soft Segment with Protected Amino in Side Chain
[0085] Put dry 0.02mol polycarbonate diol (Mn=3.5kg / mol) in a dry 500ml reaction bottle, add 300ml of anhydrous dimethylformamide, wait until it is completely dissolved, add 0.045mol of isophor Ketone diisocyanate and 0.015g triethanolamine were reacted at 75°C for 3 hours, then 0.025mol of chain extender 2,2-benzyloxycarbonyldiaminomethyl-1,3-propanediol was added to the reaction flask, and reacted for 4.5 hours. The product is precipitated in ethanol to obtain polyester polyurethane with polycarbonate diol as the soft segment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com