Preparation containing recombinant adenovirus
A recombinant adenovirus and preparation technology, applied in the field of recombinant adenovirus, can solve the problems of increasing the safety risk of gene therapy drugs, complex production, unsatisfactory stability of adenovirus, etc., to achieve easy production control and industrialization, and low manufacturing cost Low, the effect of preventing aggregation or splitting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
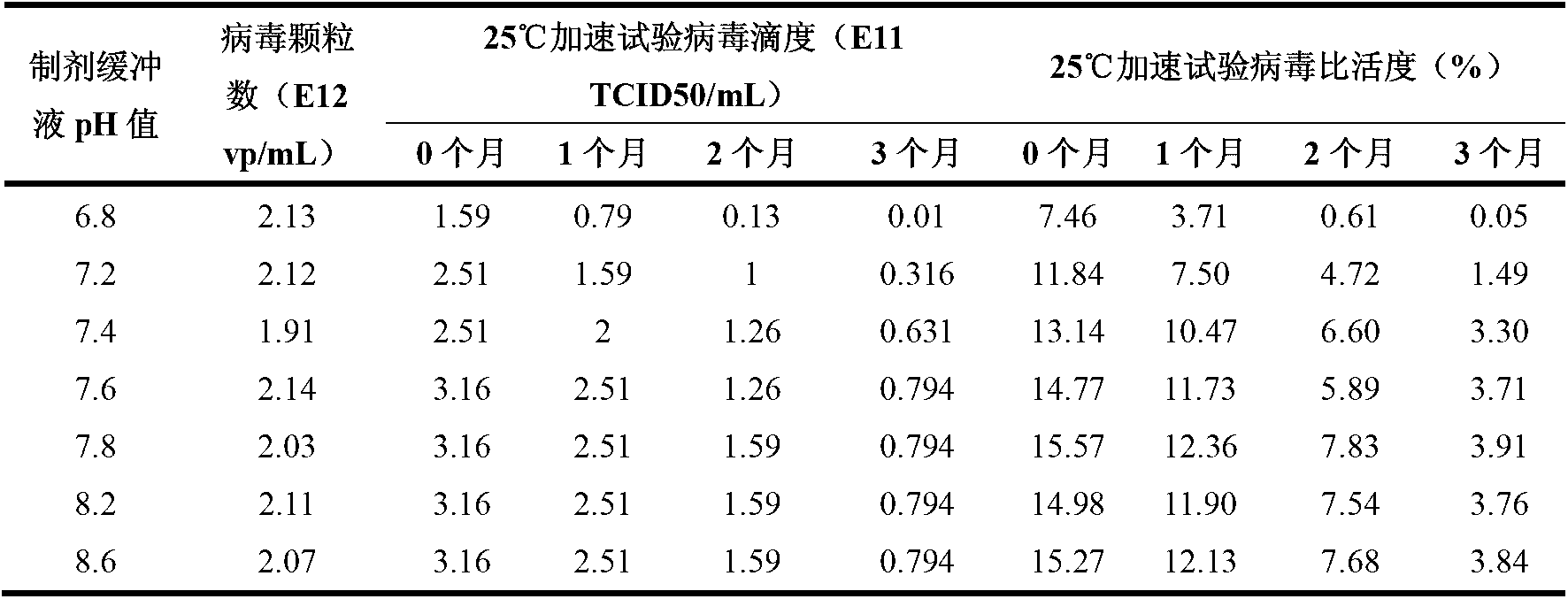
[0030] Example 1 The influence of pH value on the stability of recombinant adenovirus in the preparation
[0031] In order to study the influence of pH value on the stability of recombinant adenovirus in the preparation, under the condition of 25 ℃, we studied the pH value in the range of 6.8-8.6, and the pH value was 6.8, 7.2, 7.4, 7.6, 7.8, 8.2, 8.6. , the effect of pH on the stability of recombinant adenoviruses in preparations.
[0032] The inventors studied the effect of pH value on the stability of the recombinant adenovirus by detecting the virus titer (TCID50 / mL) and specific activity of the recombinant adenovirus in the preparation, and the results are shown in Table 1.
[0033] Table 1 Effect of pH value on the stability of recombinant adenovirus in the preparation
[0034]
[0035] NOTE: The composition of the formulation is 10 mM Tris + 10% propylene glycol (w / v), and the pH is adjusted with HCl.
[0036] It can be seen from Table 1 that when the pH is lower t...
Embodiment 2
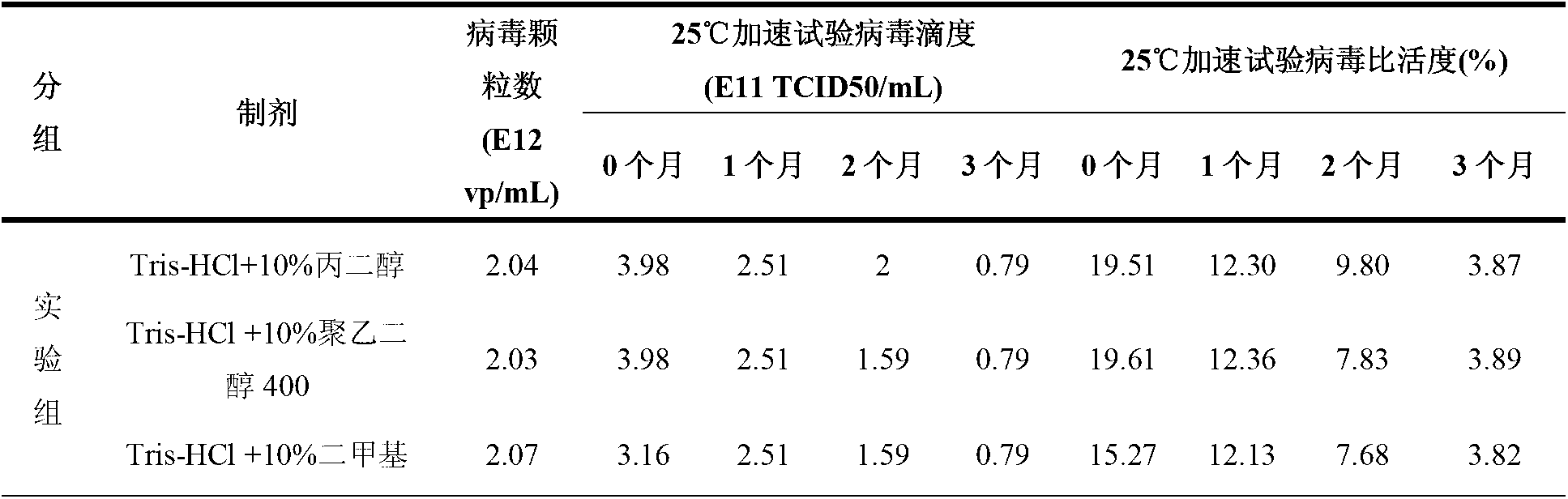
[0037] Example 2 MgCl 2 Influence on the stability of recombinant adenovirus in the preparation
[0038] In order to study MgCl 2 The influence on the stability of the recombinant adenovirus in the preparation, under the condition of 25 ℃, the present invention adopts the comparative test to study the presence of MgCl in the preparation 2 Whether it affects the stability of recombinant adenovirus. Among them, the preparation of the experimental group is the recombinant adenovirus preparation of the present invention, including recombinant adenovirus, Tris-HCl buffer and protective agents selected from propylene glycol, polyethylene glycol 400, dimethyl sulfoxide, etc., and the preparation does not contain MgCl 2 ; The preparation of the control group contains MgCl in addition to the recombinant adenovirus preparation of the present invention containing the same components and the same content 2 .
[0039] MgCl was studied by detecting the viral titer (TCID50 / mL) and specif...
Embodiment 3
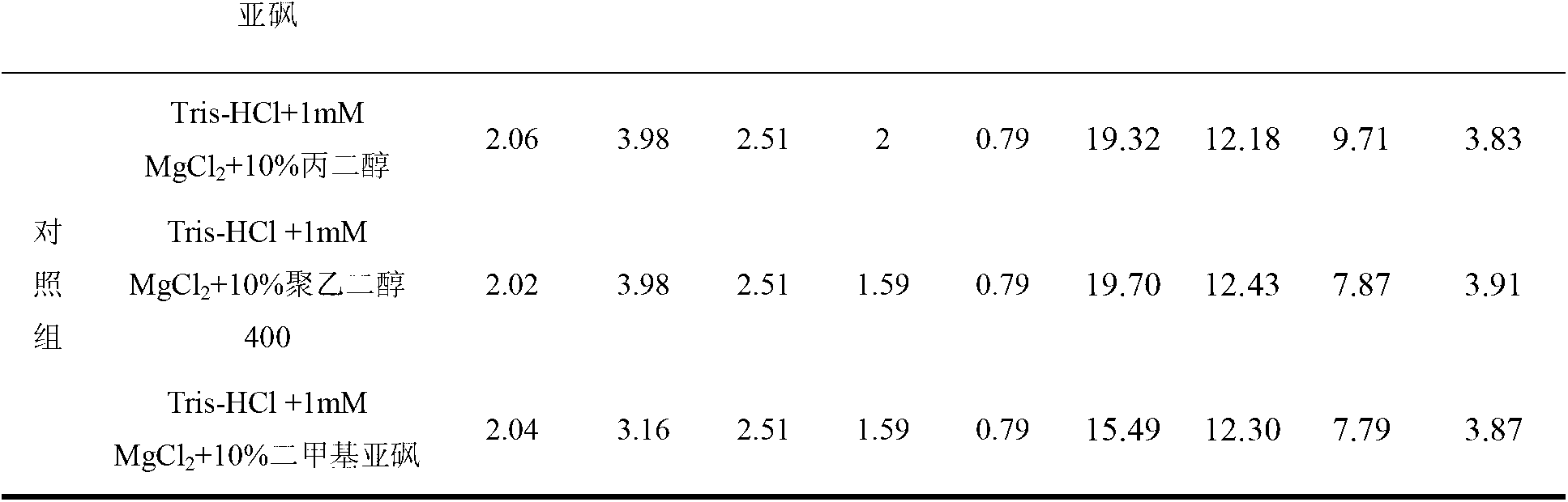
[0045] Example 3 Stability study of the recombinant adenovirus preparation of the present invention at 25°C
[0046] Under the condition of 25°C, the stability of the recombinant adenovirus in the recombinant adenovirus preparation of the present invention and the prior art preparation (ie the preparation disclosed in CN101163794A1) was studied. Among them, the preparation of the experimental group is the recombinant adenovirus preparation of the present invention, which contains recombinant adenovirus, Tris-HCl buffer and a protective agent selected from propylene glycol, polyethylene glycol 400, dimethyl sulfoxide, etc., and does not contain MgCl 2 ; The contrast preparation is the preparation disclosed by CN101163794A1.
[0047] The stability of the recombinant adenovirus preparation of the present invention at 25°C was studied by detecting the virus titer (TCID50 / mL) and specific activity of the recombinant adenovirus in the preparation. The composition and test results of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com