Preparation and solidification methods for carborane-liquid fluorine polymer
A curing method and liquid fluorine technology are applied in the field of preparation and curing of carborane-liquid fluoropolymers, which can solve problems such as low mechanical properties, and achieve the effects of low cost and simple and easy process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
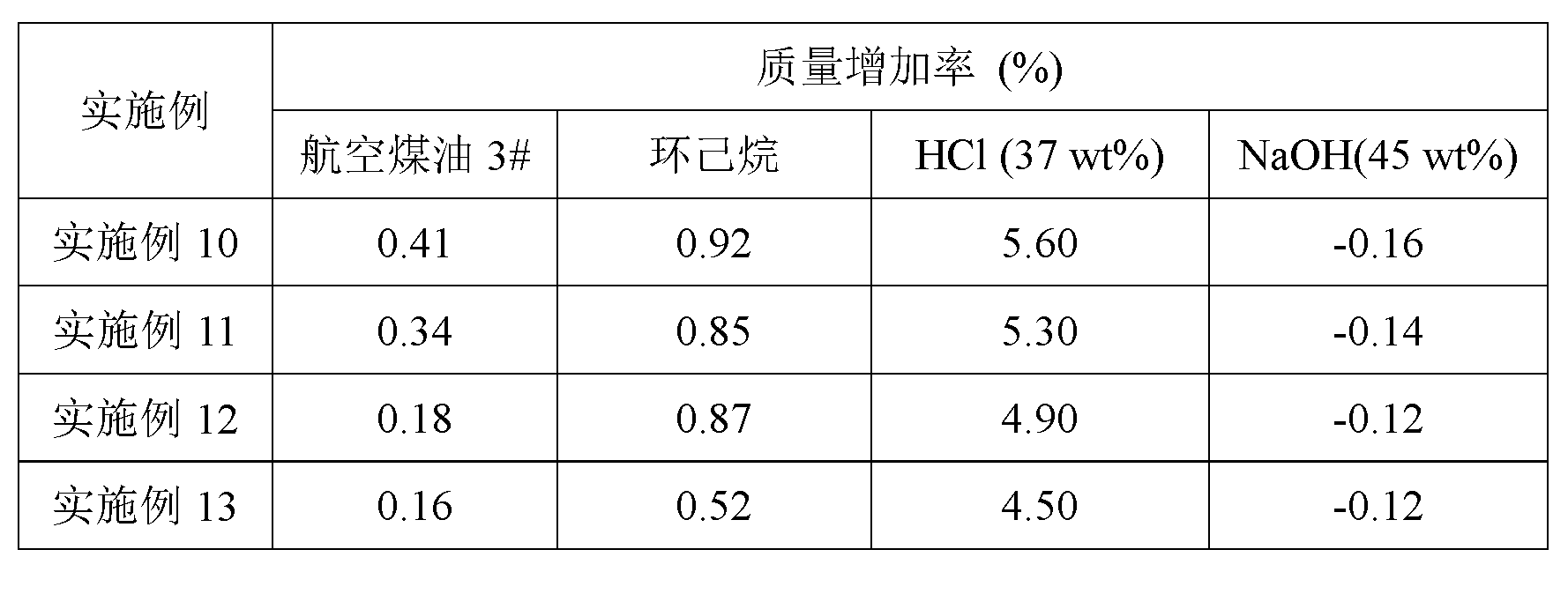
Examples
Embodiment 1
[0020] 6.27g carboxyl-terminated liquid fluoropolymer (vinylidene fluoride-hexafluoropropylene copolymer, carboxyl mass percentage content is 1.23%, number average molecular weight Mn=6.2×10 3 , weight average molecular weight Mw=11.7×10 3 ) was dissolved in 50g of tetrahydrofuran and added to a 250mL four-neck flask, followed by adding 1,7-dimethylolcarborane (0.20 g), dicyclohexylcarbodiimide (DCC, 0.5559 g), 4-bis Aminopyridine (DMAP, 0.0229 g), p-toluenesulfonic acid (TsOH, 0.0203 g), at 25 o The reaction was stirred at C for 24h. There will be white suspended matter in the system. Filter the product system to remove the white solid substance. The filtrate is separated by column chromatography and washed with petroleum ether: ethyl acetate (10:1). After 500ml of the eluent, The substance adsorbed on the silica gel was rinsed with tetrahydrofuran until the yellow color faded, the eluent was collected, the solvent was rotated, and the product was vacuum-dried at 60°C for 2...
Embodiment 2
[0023] 6.27g of carboxyl-terminated liquid fluoropolymer (vinylidene fluoride-tetrafluoroethylene-hexafluoropropylene copolymer, carboxyl mass percentage content is 2.21%, number average molecular weight Mn=2.2×10 3 , weight average molecular weight Mw=3.6×10 3 ) was dissolved in 50g of tetrahydrofuran and added to a 250mL four-neck flask, followed by adding 1,7-dimethylolcarborane (0.42 g), dicyclohexylcarbodiimide (DCC, 1.1670 g), 4-bis Dimethinopyridine (DMAP, 0.0480 g), p-toluenesulfonic acid (TsOH, 0.0426 g), at 25 oThe reaction was stirred at C for 24h. There will be white suspended matter in the system, filter the product system, remove the white solid substance, and the filtrate is separated by column chromatography, rinsed with petroleum ether: ethyl acetate eluent, after 500ml of eluent, washed with tetrahydrofuran and adsorbed on The substances on the silica gel were collected until the yellow color faded, and the eluent was collected, and the solvent was evaporat...
Embodiment 3
[0025] 6g of carboxyl-terminated liquid fluoropolymer (vinylidene fluoride-hexafluoropropylene copolymer, carboxyl content 2.14%, number average molecular weight Mn=3×10 3 , weight average molecular weight Mw=6×10 3 , the following examples all use this material, and will not be repeated) dissolved in 50g of tetrahydrofuran, added to a 250mL four-necked flask, and sequentially added 1,7-dimethylolcarborane (0.38 g), DCC (0.94 g ), DMAP (0.0229 g), TsOH (0.0203 g), at 25 o The reaction was stirred at C for 8h. The aftertreatment of the reaction system and the purification of the crude product are the same as in Example 1.
[0026] A yellow viscous liquid was obtained with a yield of 68%. The GPC characterization results of the product showed that its number average molecular weight was 4.6×10 3 , weight average molecular weight Mw=8.7×10 3 . infrared and 1 H NMR all showed that the product was a carborane-liquid fluoropolymer. The detailed results are as follows: FTIR, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com