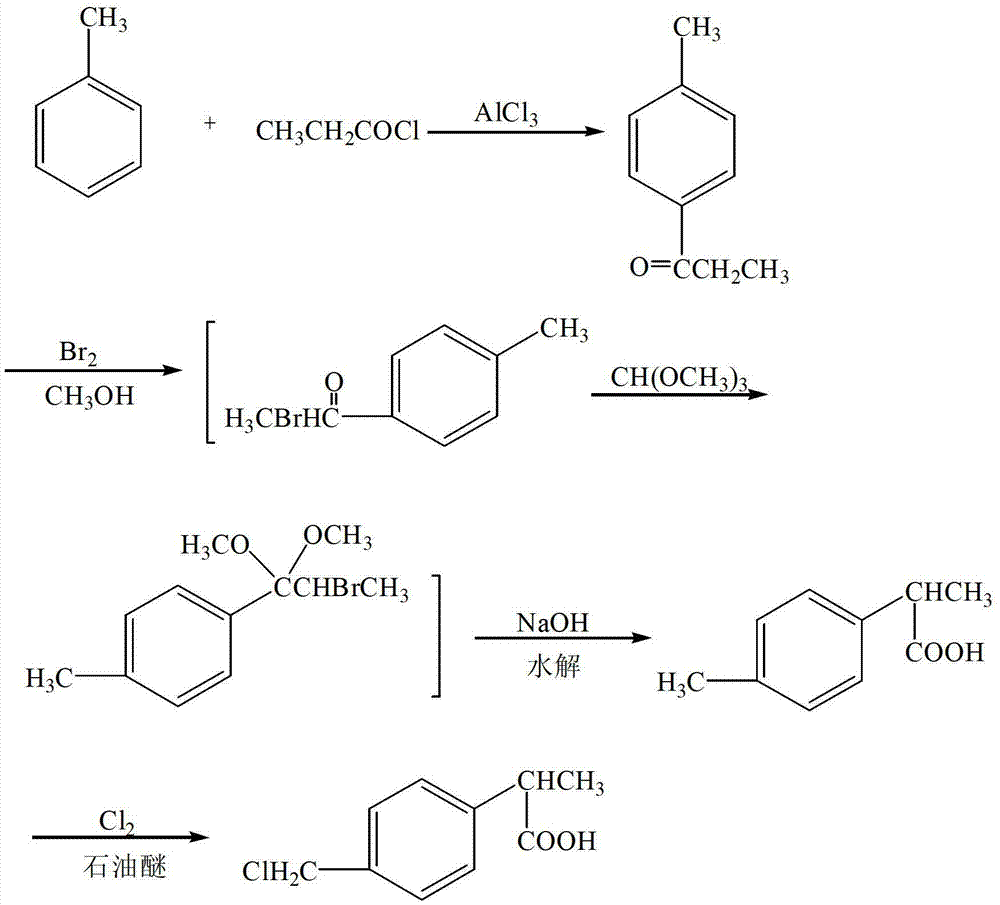
Method for preparing 2-(4-Chloromethylphenyl) propionic acid as loxoprofen key intermediate
A technology of chloromethylphenylpropionic acid and tolylpropionic acid is applied in the field of synthesis of cloxoprofen, which can solve the problems of not providing a preparation method for 2-p-chloromethylphenylpropionic acid, and achieve a short production process. , cost saving, cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Put 92g of toluene into a clean and dry reaction flask, put in 160g of aluminum trichloride when cooled to 10°C, stir for 20 minutes below 10°C, cool to 0-2°C, add 100g of propionyl chloride dropwise, and control the temperature not to exceed 5°C; After the dropwise addition, react at 0-2°C for 2 h, pour into 500 ml of ice water, filter out the solid, wash with water until neutral, and dry to obtain 110 g of p-methylpropiophenone.
[0054] Put 74g of p-methylpropiophenone into the reaction bottle, put in 300ml of methanol, add 93g of bromine dropwise at 10°C during the stirring process, raise the temperature to 60°C to react for 1h after dropping, start to heat up and distill methanol to 100°C at the same time. Cool to 70°C, add 94g of trimethyl orthoformate, stir and cool to 30°C, throw in 200ml of dichloromethane, reflux reaction at 40°C for 2h, then add 300ml of 20wt% sodium hydroxide solution, stir to heat up and evaporate at the same time Organic solvent, heat up t...
Embodiment 2
[0057] Put 92g of toluene into a clean and dry reaction flask, put in 160g of aluminum trichloride when cooled to 8°C, stir for 25 minutes below 8°C, cool to 0-2°C, add 100g of propionyl chloride dropwise, and control the temperature not to exceed 5°C; After the dropwise addition, react at 0-2°C for 2.3 h, pour into 500 ml of ice water, filter out the solid, wash with water until neutral, and dry to obtain 110 g of p-methylpropiophenone.
[0058] Put 74g of p-methylpropiophenone into the reaction bottle, put in 300ml of methanol, add 93g of bromine dropwise at 5°C during the stirring process, raise the temperature to 50°C and react for 1.3h after dropping, start to heat up and simultaneously distill methanol to 100°C . Cool to 60°C, put in 94g of trimethyl orthoformate, stir and cool to 25°C, throw in 200ml of dichloromethane, reflux at 25°C for 2.2h, then add 300ml of 20wt% sodium hydroxide solution, stir and heat up while evaporating Remove the organic solvent and heat it u...
Embodiment 3
[0061] Put 92g of toluene into a clean and dry reaction flask, put in 160g of aluminum trichloride when cooled to 15°C, stir for 13 minutes below 10°C, cool to 0-2°C, add 100g of propionyl chloride dropwise, and control the temperature not to exceed 5°C; After the dropwise addition, react at 0-2°C for 1.5 h, pour into 500 ml of ice water, filter out the solid, wash with water until neutral, and dry to obtain 110 g of p-methylpropiophenone.
[0062] Put 74g of p-methylpropiophenone into the reaction bottle, put in 300ml of methanol, drop 93g of bromine at 15°C during the stirring process, raise the temperature to 75°C and react for 0.6h after dropping, start to heat up and distill methanol to 100°C at the same time . Cool to 73°C, add 94g of trimethyl orthoformate, stir and cool to 35°C, throw in 200ml of dichloromethane, reflux reaction at 45°C for 1.6h, then add 300ml of 20wt% sodium hydroxide solution, stir and heat up while evaporating Remove the organic solvent and heat i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com