Method for preparing nano cobalt ferrite
A technology of cobalt ferrite and nano-cobalt, which is applied in the direction of nanotechnology, nanotechnology, chemical instruments and methods, etc., and can solve problems such as poor crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1) Weigh 0.1784g (0.00066mol) FeCl with a molar ratio of 2:1 3 .6H 2 O and 0.07815 g (0.00033 mol) CoCl 2 .6H 2 O, dissolved in 50ml of distilled water, fully stirred to dissolve, the metal ion concentration is 0.02M.
[0021] 2) Weigh 1.6g (0.04mol) NaOH and dissolve it in 20ml distilled water, the NaOH concentration is 2M. After stirring evenly, add N dropwise to it 2 h 4 .H 2 O (85% hydrazine hydrate) 10ml , N 2 h 4 .H 2 O concentration is 5.6 mol / L.
[0022] 3) at room temperature Down (20 o C) Stir rapidly under the condition, slowly drop the mixed solution of alkali and hydrazine hydrate prepared in step 2) into the Fe, Co salt solution, and react for 2 hours.
[0023] 4) Fully wash the black precipitate produced by the reaction in step 3) with distilled water to a pH value of about 7, then wash with ethanol 2 to 3 times, and then dry naturally or in vacuum to obtain CoFe2O4 particles.
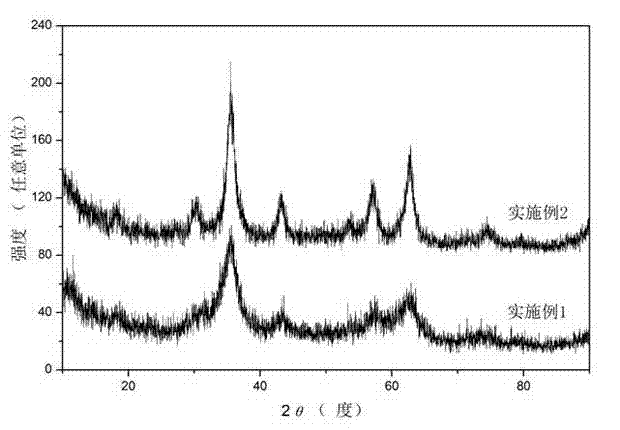
[0024]The X-ray diffraction spectrum of the sample obtained in ...
Embodiment 2
[0026] 1) Weigh 0.1784g (0.00066mol) FeCl with a molar ratio of 2:1 3 .6H 2 O and 0.07815 g (0.00033 mol) CoCl 2 .6H 2 O, dissolved in 50ml of distilled water, fully stirred to dissolve, the metal ion concentration is 0.02M.
[0027] 2) Weigh 1.6g (0.04mol) NaOH and dissolve it in 20ml distilled water, the NaOH concentration is 2M. After stirring evenly, add N dropwise to it 2 h 4 .H 2 O (85% hydrazine hydrate) 10ml , N 2 h 4 .H 2 O concentration is 5.6 mol / L.
[0028] 3) at 40 o C Stir rapidly under water bath conditions, slowly add the mixed solution of alkali and hydrazine hydrate prepared in step 2) dropwise into the Fe and Co salt solution, and react for 2 hours.
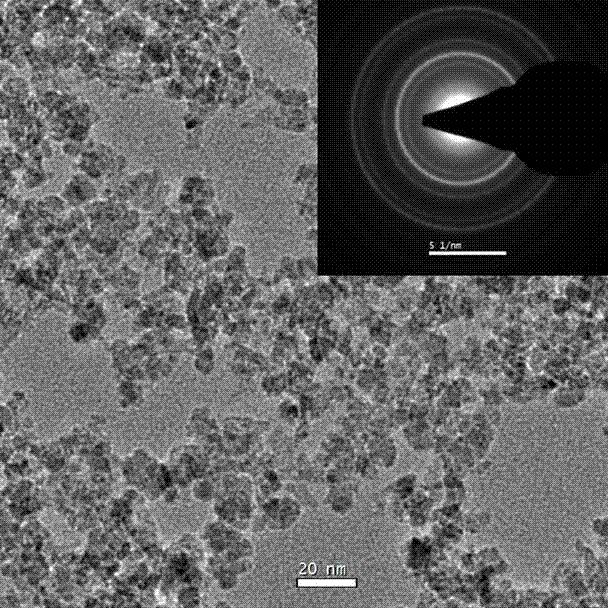
[0029] 4) Fully wash the black precipitate produced by the reaction in step 3) with distilled water to a pH value of about 7, then wash with ethanol 2 to 3 times, and then dry naturally or in vacuum to obtain CoFe2O4 particles. The average particle size is 9nm, it is superparamagnetic at room tem...
Embodiment 3
[0032] 1) Weigh 0.1784g (0.00066mol) FeCl with a molar ratio of 2:1 3 .6H 2 O and 0.07815 g (0.00033 mol) CoCl 2 .6H 2 O, dissolved in 50ml of distilled water, fully stirred to dissolve, the metal ion concentration is 0.02M.
[0033] 2) Weighing 1.2g (0.03mol) NaOH was dissolved in 20ml of distilled water, the NaOH concentration was 1.5M. After stirring evenly, add N dropwise to it 2 h 4 .H 2 O (85% hydrazine hydrate) 14ml, N 2 h 4 .H 2 O concentration is 7mol / L.
[0034] 3) at 20 o C Stir rapidly under water bath conditions, and slowly drop the mixed solution of alkali and hydrazine hydrate prepared in step 2) into the solution of Fe and Co salts, and react for 3 hours.
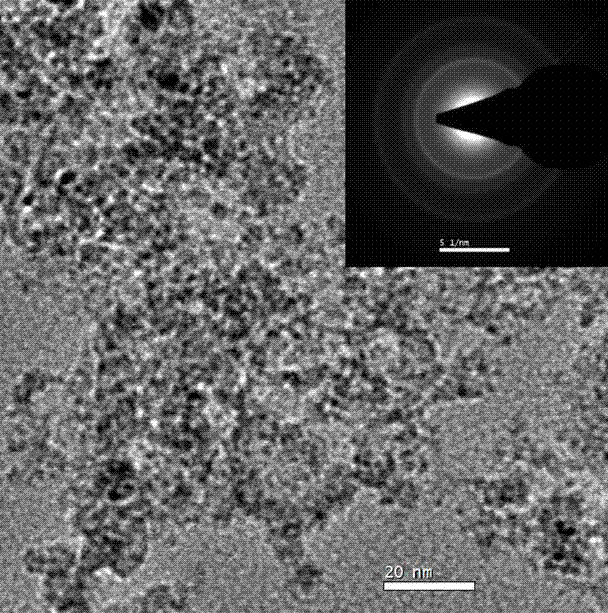
[0035] 4) Fully wash the black precipitate produced by the reaction in step 3) with distilled water to a pH value of about 7, then wash with ethanol 2 to 3 times, and then dry naturally or in vacuum to obtain CoFe2O4 particles. The average particle size is 3nm, it is superparamagnetic at room ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grain size | aaaaa | aaaaa |
| Magnetization | aaaaa | aaaaa |
| Magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com