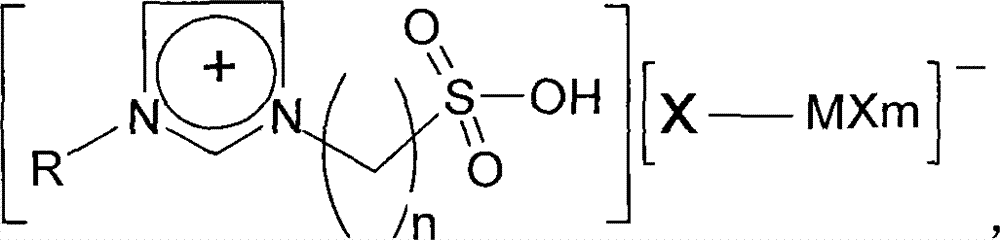
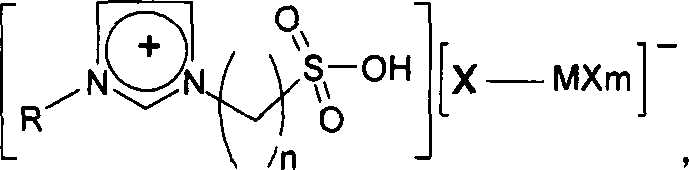
A method for preparing 5-hydroxymethylfurfural
A technology of hydroxymethyl furfural and alkyl sulfonic acid, applied in the field of product 5-hydroxymethyl furfural, can solve the problems of large mass transfer and diffusion resistance, high preparation cost, low product yield, etc., and achieve unique catalytic properties, Low three-waste emissions and stable catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: 2g sucrose, 20mL dimethyl sulfoxide, 2mL water and 0.4g ionic liquid 1-(3-sulfonic acid)-propyl-3-methylimidazolium chlorochromate (acidified inner salt and chlorinated Chromium molar ratio 1:2), was added into the autoclave, heated and stirred, and reacted at 180°C for 30min. The mass fraction of 5-hydroxymethylfurfural in the obtained product was measured by an ultraviolet spectrophotometer, and the yield of HMF was calculated to be 84.7%.
Embodiment 2
[0020] Embodiment 2: 2g glucose, 15mL n-butanol, 1mL water and 0.7g ionic liquid 1-(3-sulfonic acid)-butyl-3-ethylimidazolium ferric chloride (acidified internal salt and ferric chloride mole Ratio 1:2), added into the autoclave, heated and stirred, and reacted at 200°C for 15min. The mass fraction of 5-hydroxymethylfurfural in the obtained product was measured by a spectrophotometer, and the yield of HMF was calculated to be 48.5%.
Embodiment 3
[0022] Embodiment 3: 2g sucrose, 10mL dimethyl sulfoxide, 2mL water and 0.2g ionic liquid 1-(3-sulfonic acid)-propyl-3-propylimidazole chloride zincate (acidified inner salt and chloride Zinc molar ratio 1:2), added into the autoclave, heated and stirred, and reacted at 160°C for 60min. The mass fraction of 5-hydroxymethylfurfural in the obtained product was measured by an ultraviolet spectrophotometer, and the yield of HMF was calculated to be 65.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com